Research and improvement of pouch lithium battery shell voltage
The packaging reliability of lithium-ion pouch battery is very important to the safety performance of the battery. If there is a problem with the packaging, it will cause the battery to bulge and leak, which will seriously affect the performance and safety of the battery.

The shell voltage is to test the voltage between the positive tab and the aluminum layer of the aluminum plastic film, and its value can indicate the packaging effect of the aluminum plastic shell.
The shell voltage is divided into two types: the voltage between the positive ear and the shell, the voltage between the negative ear and the shell, when the anode shell voltage is large, the battery shell will be charged, and fire will occur during use, but it will not corrode the shell.
When the positive ear shell voltage is relatively large, it will cause corrosion of the aluminum-plastic case, causing the battery to bulge and leak. When the positive shell voltage is large, it means that the PP layer of the aluminum-plastic film has been damaged, and the aluminum layer has been in contact with the electro-hydraulic in the battery.
When the shell voltage>1.0V reaches the formation voltage of lithium-aluminum alloy, and the electronic path at the negative ear also exists, lithium ions in the electrolyte will be embedded in aluminum to form lithium-aluminum alloy, causing the aluminum shell to corrode. As the storage time increases, after the aluminum layer is corroded, moisture from the outside enters the battery, reacts with the electrolyte and active materials, generates gas, and eventually the battery bulges and leaks.
The risk of shell corrosion can be identified through the shell voltage test, but how to reduce the battery shell voltage and ensure the stability of the battery shell voltage in mass production is a difficult problem often encountered in the production process. In this paper, through the research on the law of shell voltage, starting from the mechanism of shell voltage generation, the shell voltage is improved, and the DOE research method is used to effectively improve the shell voltage level.
Shell voltage test method and experimental scheme
Case voltage test method
The case voltage is to test the voltage of the aluminum layer between the positive or negative tab and the aluminum-plastic film. This article mainly studies the voltage between the positive tab and the aluminum-plastic case. Turn the multimeter (model: UNI-T UT56 MULTIMETER) or Agilent meter (model: 34401A) to DC voltage.
After confirming that there is no abnormality in the watch, use the red test lead to touch the positive pole of the battery, and the black test lead to touch the aluminum foil in the middle layer at the edge of the case and slide slightly. The test lead needs to be in full contact with the aluminum layer in the aluminum-plastic packaging bag.
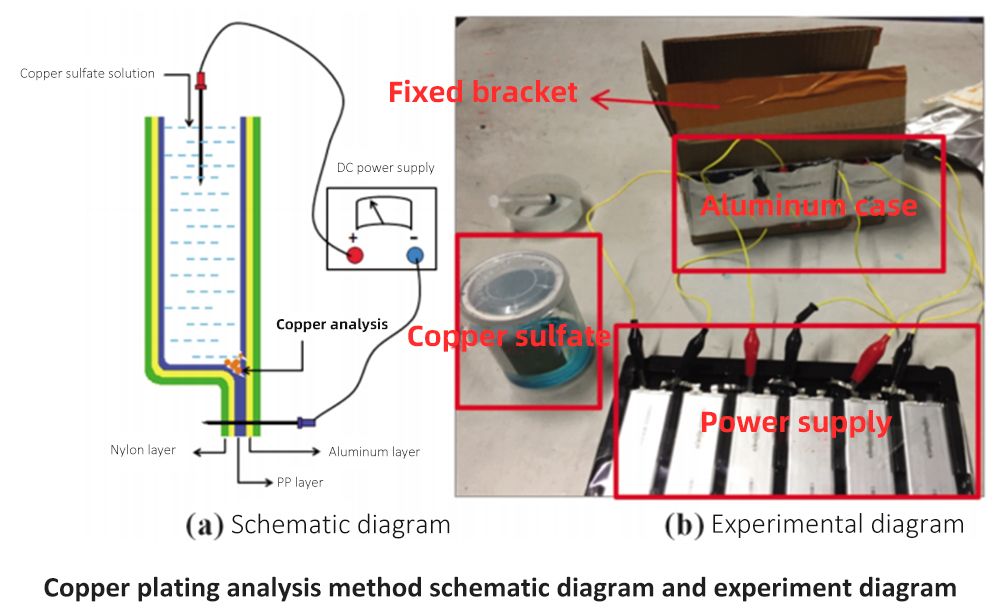
Copper plating analysis method
Cut off the leading edge of the discharged battery cell to take out the electrode group, and keep the aluminum-plastic bag; Then pour 10% copper sulfate solution into the aluminum-plastic bag, connect 3.7V DC power supply externally, the anode probe pierces the aluminum-plastic bag to make the anode conduct with the aluminum layer of the aluminum-plastic bag, and the copper sulfate solution is contained in the aluminum-plastic bag.
Immerse the positive electrode probe in the solution, and pour out the solution after testing for 20 minutes to observe the copper precipitation inside the aluminum-plastic bag. If the PP layer is damaged, the copper sulfate solution will directly contact the aluminum layer and reduce to metallic copper on the surface of the aluminum layer. The schematic diagram and experimental diagram are shown in the figure.
High power microscope test analysis method
After the experiment, the aluminum-plastic case was taken from its package, and the position to be observed was cut with a cutting machine (REM-710, made in Japan). The cross-section was observed under a VHS (VHX-6000, produced in Shanghai, China) high-power microscope to observe the sol morphology of the PP layer, the absence of the PP layer, and the position of copper precipitation.
Experimental scheme
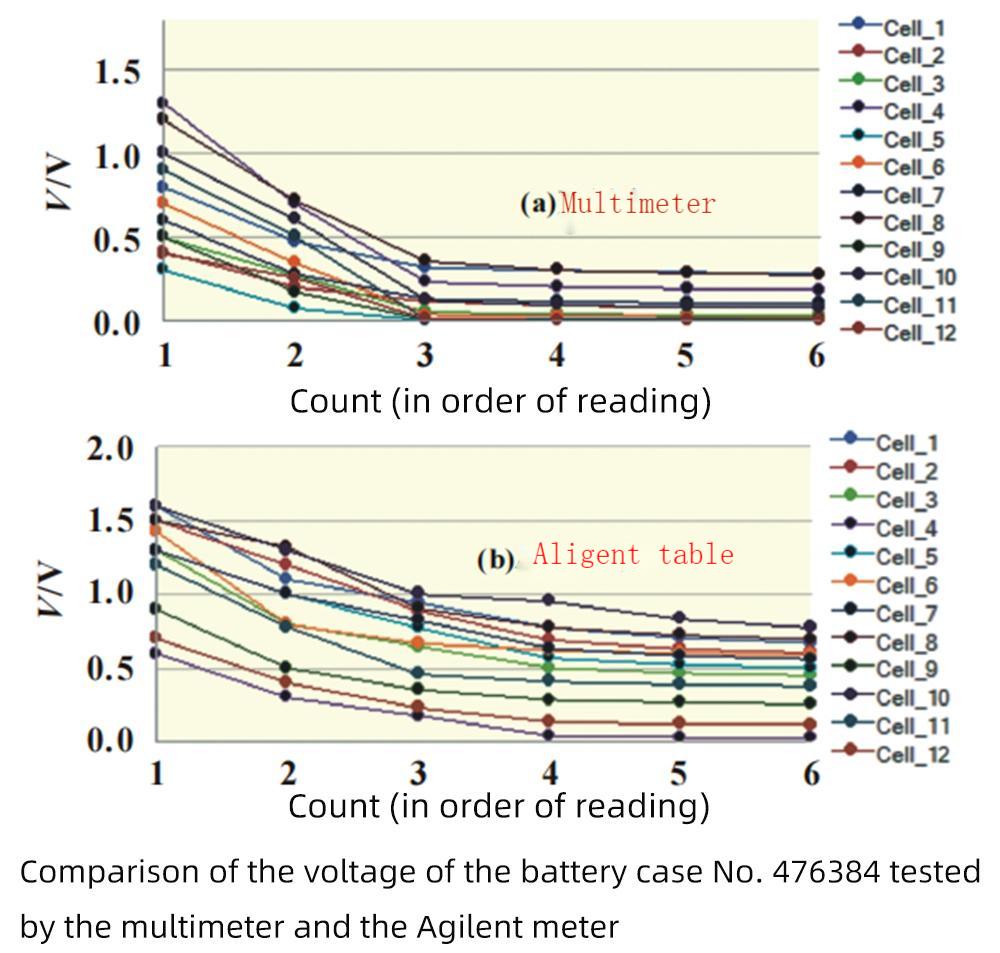
Take two groups of 616072 and 476384 pouch polymer batteries, 24 batteries in each group, and test the case voltage with a multimeter and an Agilent meter respectively. Use the video to record the whole reading change, and then read all the displayed values in sequence in slow motion to study the change law of the shell voltage;
Use a multimeter to test the case voltage of batteries with different processes and different folding methods, and study the variation of case voltage with time and folding methods; Use a multimeter to select batteries with a case voltage <0.8V, 0.8~1.0V, 1.0~1.2V, 1.2~2.0V, 2.0~3.0V, and >3.0V for electroplating to analyze its risk. Cut and analyze the position of copper precipitation, study its cause, and analyze the improvement method.
Specific analysis of shell voltage
Variation law of shell voltage
The picture is a comparison of the voltage of the 476384 battery case tested by the multimeter and the Agilent meter. The first value is the largest, then drops rapidly, and gradually stabilizes from the third value. The results of the Agilent test are significantly larger than those of the multimeter.
Theoretically, the aluminum layer between the cathode or the anode and the aluminum-plastic film is insulated, and the shell voltage should be 0V; However, in the actual process, the aluminum-plastic film will be partially damaged, resulting in local conduction of the aluminum layer between the cathode and anodes and the aluminum-plastic film (ion channels and electron channels), forming a micro-battery, and thus a potential difference (voltage).
Ion channel: During the cell packaging process, the PP layer at the edge sealing position is more likely to be damaged after being heat-pressed. In addition, the edge folding process is also likely to cause damage to the PP layer.
Electronic channel: the aluminum layer of the aluminum-plastic film is in contact with the nickel tab or anode.
At the beginning of contact, the concentration of ions is the highest, and the polarization is also the largest, so the test value is the highest; as the ion diffusion gradually tends to balance, the test value of the shell voltage decreases rapidly and tends to be stable.
Reproducibility of shell voltage
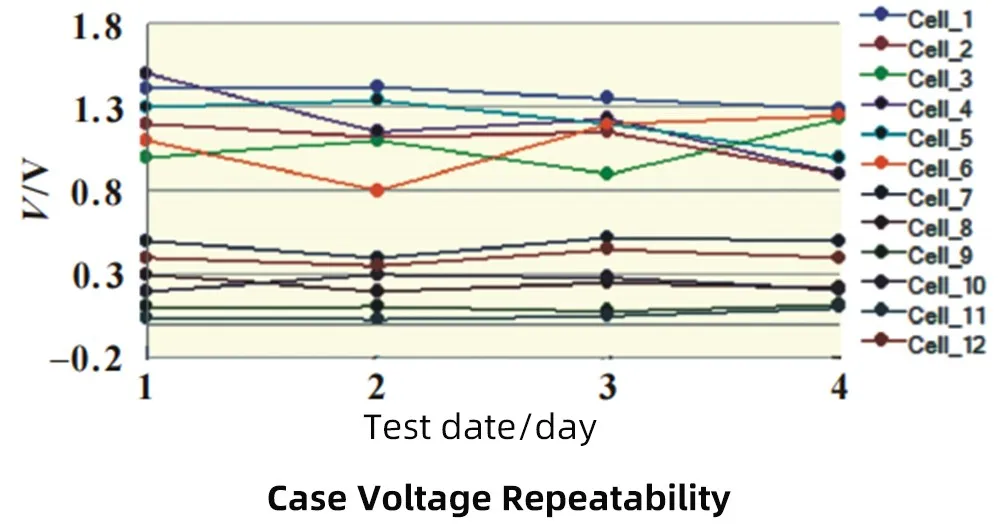
Twelve 616072 cells were randomly selected, and the case voltage value was measured and recorded with a multimeter. Continuous monitoring was carried out for 4 days to investigate the repeatability of the test value.
The sample results show that the reproducibility of the shell voltage is poor, because the shell voltage is generated because the aluminum layer exposed at the damaged position of the PP layer and the positive/negative tab form an ion channel through the electrolyte, therefore, the result of each test is closely related to the ion concentration concentration, the folding time of each battery, as well as the degree of folding, test time, and test contact methods may lead to inconsistent ion concentrations, so there are slight differences in the results of each test.
Electroplating analysis of different shell voltages
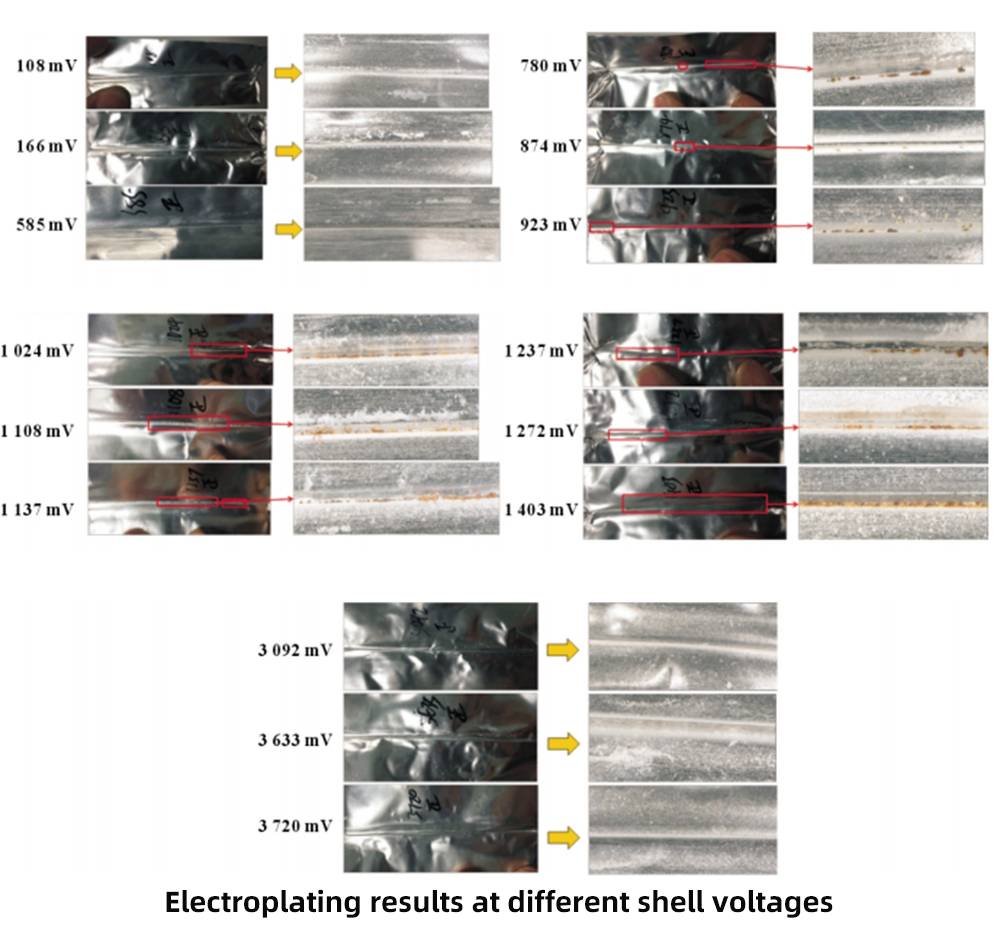
Use a multimeter to select 3 batteries of each gradient shell voltage of the 616072 model, and disassemble them for copper plating experiments. In the figure, for the samples with case voltage <0.8V, there was no copper deposition phenomenon in the electroplating experiment, which shows that the package with case voltage <0.8V is more reliable.
The three samples with case voltage between 0.8 and 1.0V gradually showed spot-like copper deposition phenomenon. It shows that the PP layer has been slightly damaged at this time, and the battery with a case voltage of 1.0~2.0V has dense and continuous copper precipitation phenomenon, which shows that the risk of case corrosion is relatively high in this case.
No shell voltage of 2.0~3.0V was found in production; No copper precipitation was found in batteries with a case voltage > 3V. In this case, it is inferred that the electronic channel has been connected, that is, the case voltage is relatively high due to the unpackaged anode tab.
Experiments have shown that the risk of shell corrosion is the lowest for batteries with a shell voltage of less than 0.8V. When the shell voltage is between 0.8 and 2V, the risk of corrosion increases with the increase of the shell voltage. When the shell voltage is greater than 3.0V, the electronic channel is connected, and the risk of corrosion is the highest.
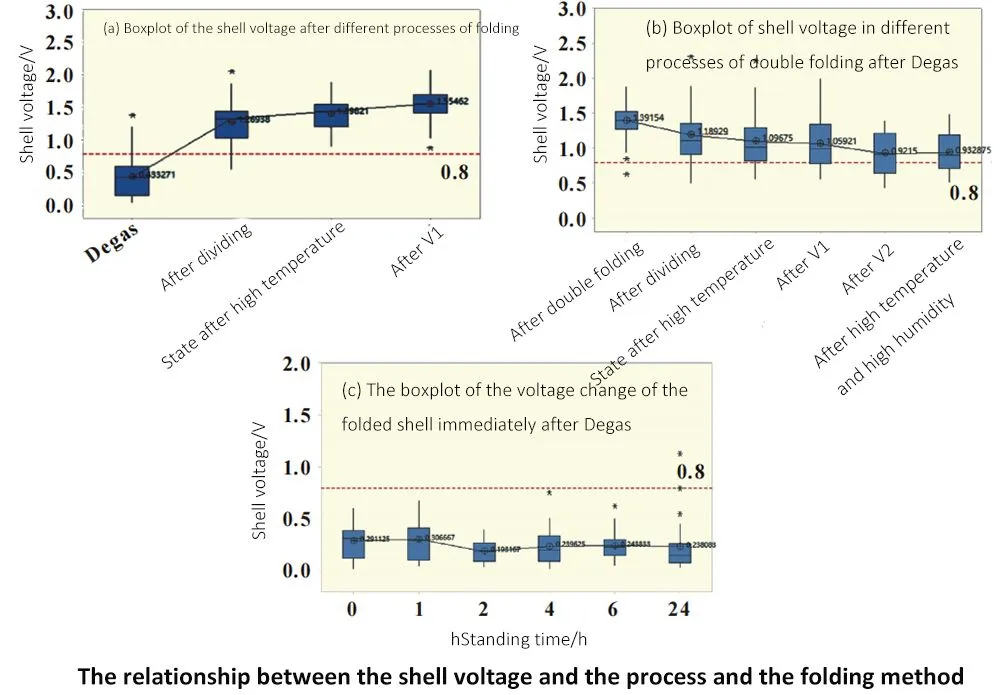
Variation of shell voltage with time
Randomly select two groups of 616072 type batteries in the same batch and the same process, and design two groups of experiments: Group 1, take out the cell sample and fold it first, then test the case voltage value and put it back into the original batch, and then take the group of samples to test the case voltage value and record it after each process;
For group 2, refer to the sampling process of group 1. Each process takes an equal amount of samples from the same batch of batteries, first folds the edges, and then tests the case voltage value and records it. It can be seen from (a) and (b) in the figure that according to the experimental method of group 2, the shell voltage value is gradually increasing; However, according to the experimental method of group 1, the voltage value of the battery case after folding gradually decreases with time.
According to the experimental results, there are two possibilities for the mechanism: Inference 1, the internal electro-hydraulic penetration has not been balanced after Degas, and the shell voltage is low. After standing for a sufficient time, the electro-hydraulic penetration reaches equilibrium, and the shell voltage increases;
Inference 2, the PP layer at the back edge of Degas is not completely cooled, the tensile performance is good, it is not easy to be damaged, and the shell voltage is low. After standing still, the tensile properties of the PP layer will deteriorate after complete cooling, and the PP layer will be easily damaged after folding, so the shell voltage is high.
In order to verify the above two inferences, the following experiment was carried out: take 24 batteries after Degas, immediately fold the edge and record the voltage value of the case, and then continuously monitor the change of the case voltage value, the result is shown in (c).
As the standing time increases, the shell voltage does not change significantly, and the rising trend in (a) and (b) in the figure does not appear, so the mechanism of inference 2 is considered to be in line with the experimental law.
Principle of shell voltage generation and battery corrosion mechanism
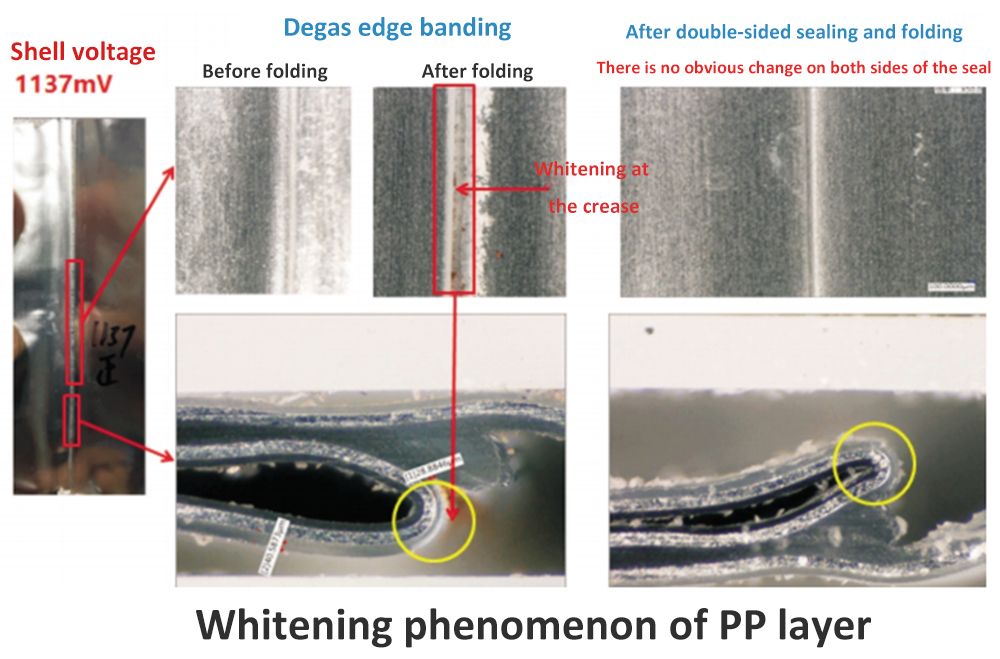
By gradually cutting the copper precipitation position of the aluminum-plastic bag, the position where the copper particles are almost close to the aluminum layer (distance 4mm) can be found, but no obvious damage point of the PP layer can be found. It can be inferred that the damage of the PP layer is not macroscopic, but micropores are formed inside the PP layer, connecting the free electrolyte and the aluminum layer.
There is a significant difference in the morphology of the PP layer at the packaging position before and after folding, and the whitening phenomenon of the PP layer at the crease position can be clearly found.
However, the cell with qualified shell voltage does not have this phenomenon. This phenomenon indicates that the mechanical properties of the PP layer after packaging have changed. After the edge is folded, tiny channels appear inside it, causing the electrolyte to come into direct contact with the aluminum layer, thereby creating a shell voltage.
Damage to the PP layer will cause the electro-hydraulic to contact the aluminum layer. When the voltage is greater than the formation voltage of lithium-aluminum alloy, and at the same time, when the electronic pathway at the tab also exists, a tiny electrolytic cell will be formed, and lithium ions in the electrolyte will be embedded in the aluminum layer to form a lithium aluminum alloy;
The formation of lithium-aluminum alloy leads to the destruction of the aluminum lattice and the damage of the aluminum layer. In severe cases, the aluminum layer will be corroded and penetrated. The nylon layer cannot block water molecules, causing the external water to react with the electrolyte to produce hydrofluoric acid; the hydrofluoric acid intensifies the erosion of the aluminum layer, resulting in the extension of the damaged area of the aluminum layer.
The figure verifies the corresponding phenomena of the whitening of the PP layer and the shell voltage and the copper precipitation. When the battery is folded in the forward direction, the copper precipitation position is on the side with a greater degree of bending and stretching. Copper precipitation did not appear on the side with a smaller bending degree. Similarly, copper precipitation appeared on the side with a greater bending and stretching degree during reverse folding, and copper precipitation did not appear on the other side.
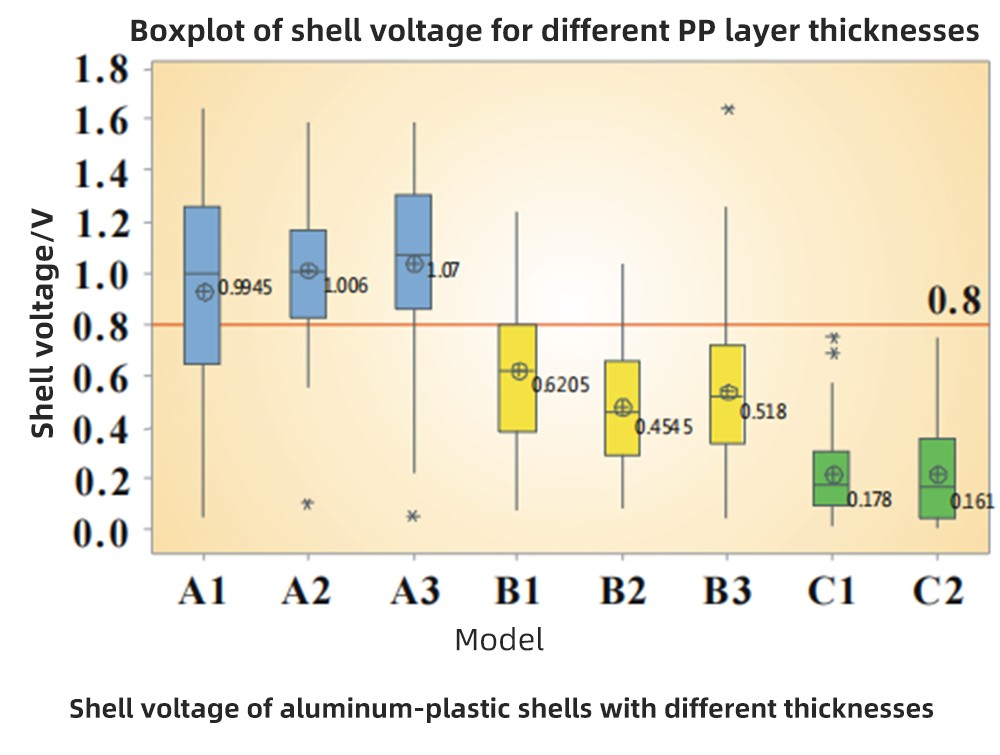
Shell voltage of different PP layer thicknesses
In the figure, the shell voltages of 91, 113 and 158mm aluminum-plastic shells are used respectively. A single packaging machine is used for packaging successively. After packaging, the test case voltage results are as follows: blue is 91mm aluminum plastic, yellow is 113mm aluminum plastic, and green is 158mm aluminum plastic.
The thicker the aluminum-plastic case, the thicker the PP layer. For aluminum-plastic cases with different PP layer thicknesses, the same packaging parameters are used. The thicker the PP layer, the lower the case voltage. When the battery packaging parameters are the same, the PP layers that can be dissolved are the same. For the aluminum-plastic case with a thick PP layer, it only destroys or changes the surface of the PP layer, and the PP performance of the deeper part does not change, which can play a normal role in isolating the electrolyte, so the case voltage is low.
Improvement of shell voltage
According to the above mechanism, we can use two ways to improve the shell voltage:
● By improving the performance of the PP layer, improving its tensile properties or increasing its thickness, the shell voltage can be improved;
● Optimizing the process parameters and reducing the stretching of the PP layer can improve the shell voltage. Through verification in production, the experimental effect is obvious, and the shell voltage can be significantly improved.
Conclusion
Through this study it is shown that:
● In the continuous test of shell voltage, the first value is the largest, and the latter gradually tends to be stable, and has a great relationship with the reading accuracy of the measuring equipment. The shorter the reading interval of the equipment, the greater the measurement result;
● The reproducibility of the shell voltage is relatively poor, and the test results of the same battery are quite different for several times;
● The battery with case voltage less than 0.8V has no risk of swelling and leakage, before 0.8~2.0V, with the increase of the shell voltage, the risk of swelling and leakage gradually increases, which is caused by ion pathways, shell voltage greater than 3.0V is caused by electronic pathways;
● The mechanism of the shell voltage is that the mechanical properties of the encapsulated PP layer are reduced, and the stretching during the folding causes the PP layer to generate fine channels, forming an ion path for the electrolyte to contact the aluminum layer, and the shell voltage is generated.By optimizing the process parameters, or increasing the thickness of the PP layer, and changing the properties of the PP layer, the voltage of the battery case can be significantly improved.
If you want to know about pouch battery-related comparison articles, please refer to pouch cells vs cylindrical, prismatic vs pouch cells and more voltage-related articles below.