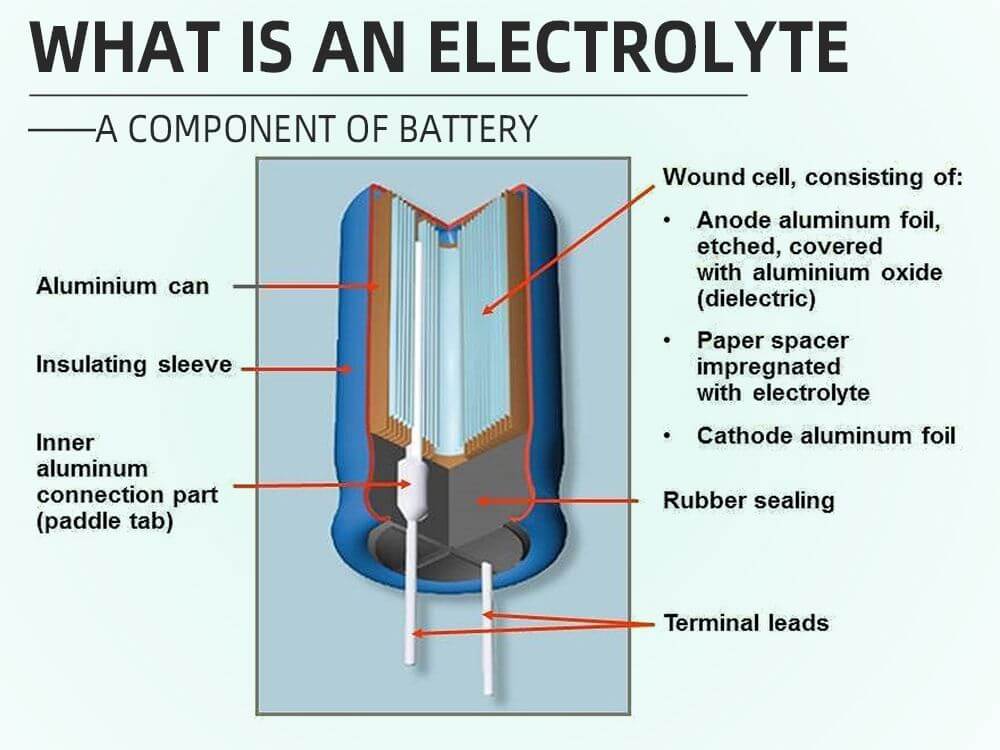
What is an electrolyte - a component of battery

What is an electrolyte in battery – definition of battery electrolyte
The electrolyte is a conductive media made from solid, gel, or fluid materials. Electrolyte is a necessary part of a battery, if the battery does not have an electrolyte, it cannot generate electricity.
An electrolyte supports the movements of ions between anode and cathode in a battery. This article will further explore what is an electrolyte.
What is electrolyte in a battery made of
The electrolyte is a blend of lithium hexafluorophosphate salt solution in popular lithium batteries. Different types of batteries tend to have different electrolytes. Generally speaking, electrolyte substances in batteries are divided into soluble salts, additives and solvents.
What is an electrolyte in different batteries? Following is the answer:
- The electrolyte of a solid-state battery exists in the form of a polymer.
- The electrolyte of a nickel-cadmium battery is potassium hydroxide, an alkaline electrolyte.
- The electrolyte of a NiMH battery is potassium hydroxide or a similar substance.
- In lithium-ion batteries, the conventional electrolyte is liquid, and there are also solid or colloidal electrolytes, which are often referred to as polymer cells. Our website organizes top 10 ithium ion battery electrolyte company and helps to understand the development of lithium-ion battery electrolytes.
- In lead acid batteries, diluted sulfuric acid is the electrolyte.
What types of electrolytes are there?
What is an electrolyte in lithium battery? In general, five types of electrolytes are used in lithium batteries: aqueous solutions, solid state electrolytes, polymer electrolytes, ionic liquids, and hybrid electrolytes.
According to the electrolyte used, it can be divided into: high temperature molten salt lithium battery, organic electrolyte lithium battery, non-aqueous electrolytic inorganic lithium battery, solid electrolyte lithium battery and lithium battery.
What are commonly used electrolytes
The electrolyte of alkaline batteries commonly used in daily life is potassium hydroxide. In a lithium battery, its electrolyte is a lithium salt compound called lithium hexafluorophosphate.
How do electrolyte work in a lithium-ion battery
Like all batteries, in lithium batteries, electrolytes work as a conductor, allowing the movement of ions between the cathode and anode during charging and discharging. In a lithium-ion battery, the ion moves from the cathode to the anode, and during recharge, the ion moves back.
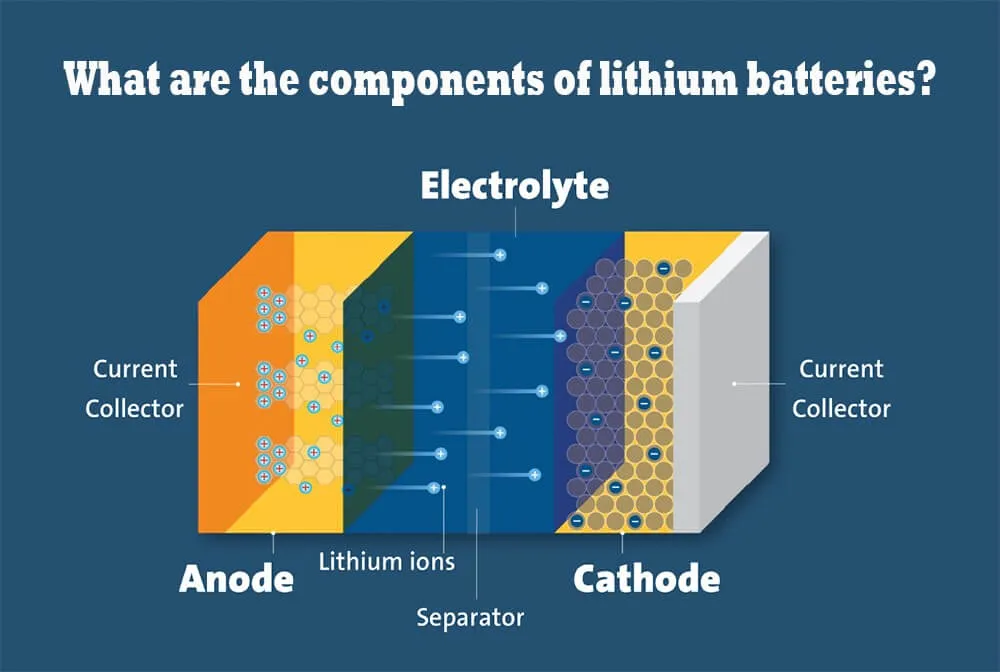
What are the components of lithium batteries
Excluding the outer shield, a lithium battery has four main components: the anode, cathode, separator, and electrolyte. Each has its functions.
Anode: During the charging, the lithium-ion gets stored in the anode. When the battery is connected to a power consumption device or connected with a wire for power utilization, the anode releases the current to pass through the external circuit.
Cathode: The cathode material of the lithium ion battery is a lithium compound. It is a source of lithium ions, which gain electrons from an external circuit and are reduced in an electrochemical reaction.
Separator: It is positioned between the anode and cathode. The separator stands like a wall and safeguards the battery function. What is an electrolyte role? It prevents the electron flow and regulates by letting only the ion flow through the separator and maintaining the battery’s electrochemical condition.
Electrolyte: The electrolyte is an important part of the battery, which plays the role of transporting ions and conducting current between cathode and anode. What is electrolyte for lithium battery is a compound of salt, organic liquids, and additives. The battery electrolyte enables the ion to move between the electrodes without letting the electrons flow.
How do lithium batteries work
Understanding how lithium batteries work can help us understand more fully what is electrolyte.
When the battery charging, lithium ions are extracted from the lithium-containing compound of the cathode, and the lithium ions move to anode through the electrolyte. The carbon material of anode has a layered structure, and it has many micropores. The lithium ions reaching anode are embedded in the micropores of the carbon layer. The more lithium ions embedded, the higher the charging capacity.
When the battery is discharged (that is, the process in which we use the battery), the lithium ions embedded in the carbon layer of anode come out and move back to cathode. The more lithium ions returned to cathode, the higher the discharge capacity. The battery capacity we usually refer to is the discharge capacity. During the charging and discharging process of lithium ion batteries, lithium ions are in a state of motion from cathode to anode to cathode.
Is electrolyte in lithium batteries safe
Yes, the electrolyte in a lithium battery is safe to use.
There might have been fire problems in the early stage of lithium batteries due to overheating of the electrolyte solvent. With the introduction of the Battery Management System, this issue has been appropriately addressed, and the new generation lithium batteries are very safe to use. The colloidal electrolytes are now used, which are safer and less likely to catch fire.
How do electrolytes affect battery performance
Since electrolytes are a conductor and help the movement of ions from the cathode to the anode and flow back to the anode, a good electrolyte quickly supports the flow of the ion movement. For better and efficient ion movement facilitation, the electrolyte should have the metal element in both halves of the cell or one half. It will help the ion-molecule absorption quickly.
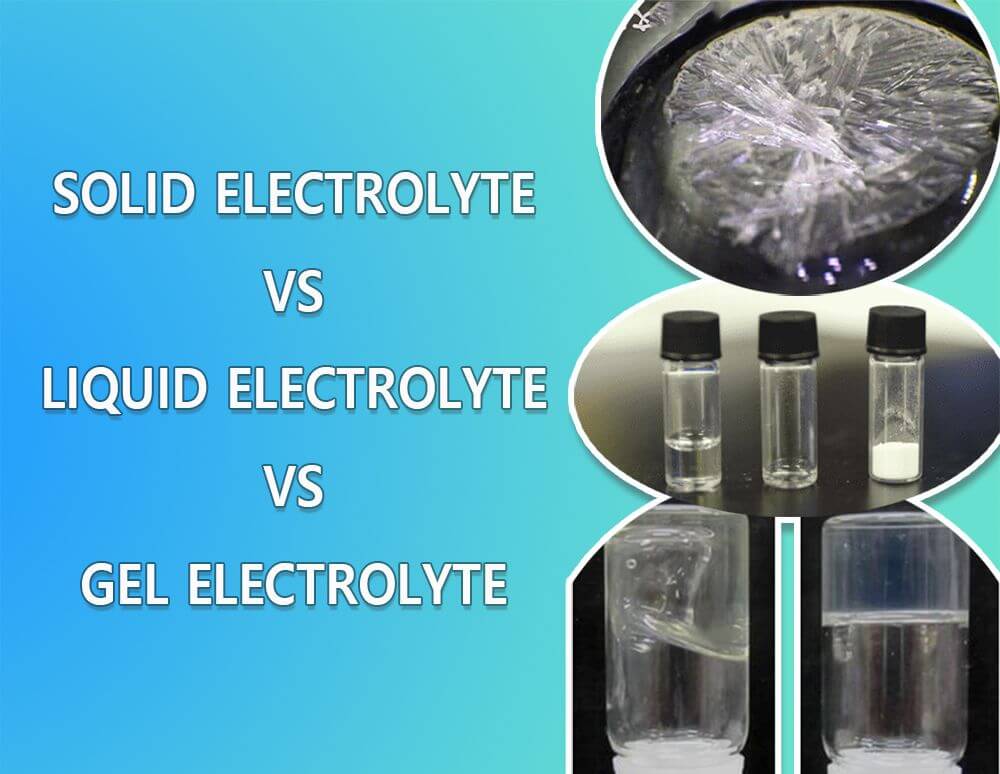
Solid electrolyte vs liquid electrolyte vs gel electrolyte
- Solid electrolyte: It is a solid conductor letting the ion movement in a solid-state battery with liquid or gel electrolyte functional characteristics. The solid state electrolyte is much safer than any other form of electrolyte. It will not leak. Batteries with solid electrolytes will have excellent thermal and mechanical stability. They have low inflammability, low discharge rate, are highly recyclable, and have low power density storage characteristics.
- Liquid electrolyte: Liquid electrolyte is an aqua form of an electrolyte comprising a minimum of one salt and one liquid non-aqueous polar additive. The solvent can be water, and salt or acid can be the ionizable substance. Lead-acid batteries use liquid electrolytes.
- Gel electrolyte: The gel electrolyte is similar to a solid electrolyte and liquid electrolyte, capable of acting like a conductor allowing the movement of ions between the anode and cathode. It is a blend of liquid electrolytes and solid polymers.
Comparison table below explain what is an electrolyte in terms of pros and cons for different types.
| Electrolyte types | Advantages | Disadvantages |
|---|---|---|
| Solid electrolyte |
● High storage capacity ● Can store high energy ● No dendrites ● Excellent electrochemical stability from 0 to 5V ● Long life cycles ● No leakage ● No spilling ● No boiling ● It can operate between -40°C to 150°C |
● Expensive ● Electrochemical stability issues with some solid electrolytes. ● Not commercially available. |
| Liquid electrolyte |
● High conductivity ● During charge and discharge, it can accommodate the size changes of electrodes. ● Suitable for high-capacity electrodes. |
● Electrochemical instability ● Low ion-selectivity. ● Potential risk due to electrochemical instabilities. ● Inexpensive |
| Gel electrolyte |
● Can process easily. ● Excellent safety. ● Low weight. ● Excellent mechanical flexibility. ● Maintenance free ● No leakage ● Excellent vibration resistance. |
● They are relatively expensive. ● Slow recharging. |
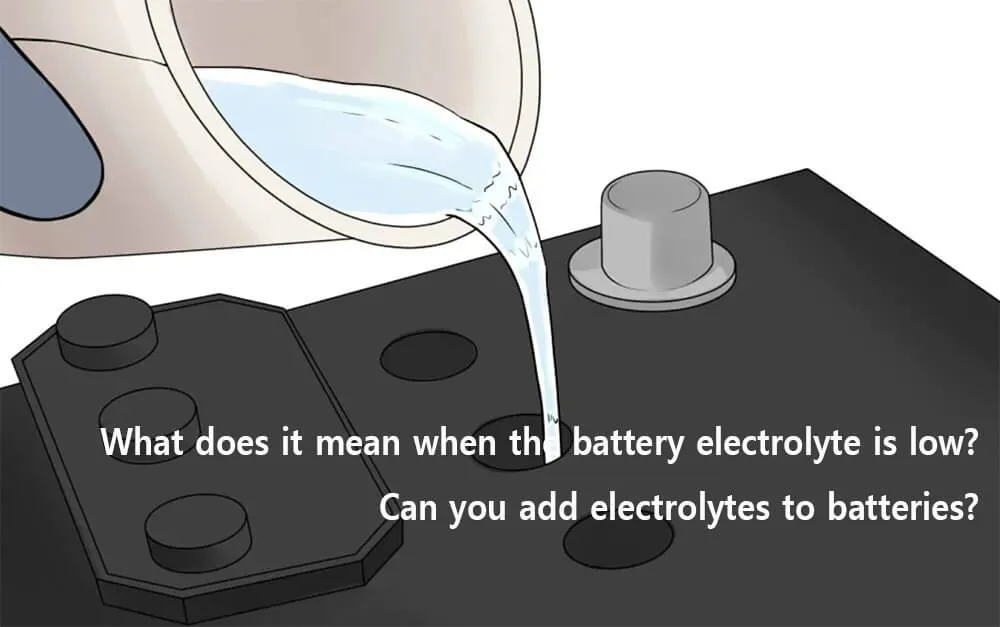
What does it mean when the battery electrolyte is low?
When the electrolyte battery becomes low, its capacity will become low, and it cannot recharge to the designed level or deliver the energy for an extended period.
However, most of this happens with conventional lead-acid batteries. The battery is not sealed and requires water to be added when the electrolyte is depleted. Therefore, regular maintenance is required. This is not the case with lithium-ion batteries, which are completely sealed and require no maintenance.
You can add electrolytes to a battery only if it is serviceable. Do not add electrolytes to the sealed battery. You can add electrolytes only to a non-sealed acid battery. You can manually check the electrolyte level in an acid battery by opening the acid lid. If the electrolyte level is low, you need to add battery water to increase the electrolyte level.
How to make a battery electrolyte solution
You can make electrolyte solutions at home using sulfuric acid and water for acid batteries. The best mixing proportion is one part of sulfuric with 10 parts of water, allowing it to dilute properly. After dilution, you can transfer the acid water to the battery chamber.
What is an electrolyte and how to make? For making electrolytes for lithium vs alkaline batteries, you must use an element used as one-half or both half cells as an electrolyte, along with powerful acid and salt that can augment the flow of ions between the cathodes.
Conclusion
As per the present technology, all batteries, whether lithium, alkaline, silver dioxide, acid, or other batteries, should have electrolytes to carry out the movements of ions from cathode to anode, and in recharge batteries, the ions will recourse to the cathode.
The electrolyte must be a conductor for quick movements of ions between the electrodes so that the battery can charge quickly. Apart from the cathode, anode, and separator, the electrolyte is an integral part of the battery. We can understand What is an electrolyte if we know the role of electrolytes in power storage.