Characteristics and application comparison of car battery types

The role of car batteries and car battery types
In electric vehicles, the power battery serves as the power source of the entire vehicle and provides power for the vehicle. The performance of the car battery types directly determines the actual value of the car. The function of the power battery is to receive and store the high-voltage direct current provided by the on-board charger, generator, braking energy recovery device or external charging device, and provide high-voltage direct current for electric vehicles.
The main car battery types include lead-acid batteries, nickel metal hydride batteries, lithium-ion batteries, fuel cells, and supercapacitors. The following are the characteristics and application comparison of car battery types:
| Types | Characteristics | Application |
|---|---|---|
| Lead-acid batteries | Low cost, mature technology, low single energy and power, bulky, high degree of commercialization | Ordinary cars, low-voltage batteries for new energy vehicles, high-voltage power batteries for electric bicycles |
| Nickel metal hydride batteries | Better security, longer life, high cost, high degree of commercialization | Toyota hybrid car |
| Lithium-ion batteries | High energy density, low self-discharge rate, long service life, high cost, high degree of commercialization | Most electric vehicles |
| Fuel cells | High energy density, large room for improvement, high cost, low degree of commercialization | Electric commercial vehicle |
| Supercapacitors | Fast charging speed, high efficiency, fast discharging efficiency and durable charging | Electric vehicle energy assistance |
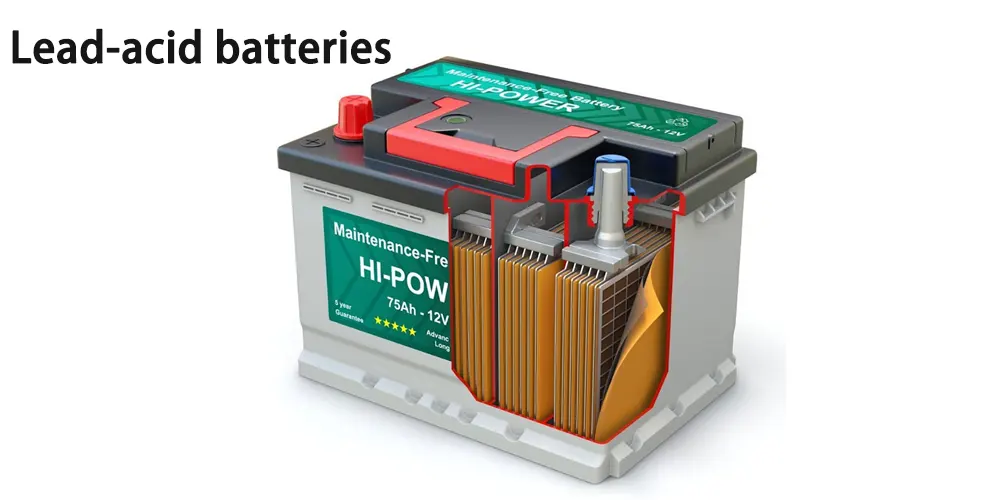
Lead-acid batteries
Among the car battery types, lead-acid batteries are widely used as a starting power source for internal combustion engine vehicles. It is also a mature electric vehicle battery with good reliability, easy access to raw materials, and low price; its specific power can basically meet the power requirements of electric vehicles.
But it has two major disadvantages: one is low specific energy, the mass and volume occupied are too large, and the mileage of one charge is short; the other is short service life and high cost of use. At present, lead-acid batteries are no longer used as power batteries.
Nickel metal hydride batteries
In the car battery types, nickel-metal hydride batteries are synthesized by hydrogen ions and metal nickel.
● Advantages: Large battery energy reserve, lighter weight, longer service life, and no pollution to the environment.
● Disadvantages: The manufacturing cost is too high, and the performance is worse than lithium batteries.
Representative models are: Toyota Prius, Ford Escape and so on.
Lithium-ion batteries
Lithium ion battery refers to a type of battery that uses lithium element as the main active material in the electrode material, mainly including lithium metal battery and lithium ion battery. The lithium batteries mentioned in this article are mainly lithium-ion batteries.
As the best rechargeable batteries with high voltage and high energy density, lithium-ion secondary battery has the following outstanding features in the car battery types: light weight, large energy storage, no pollution, no memory effect, and long service life.
In the case of the same volume and weight, in the car battery types, the storage capacity of lithium batteries is 1.6 times that of nickel-metal hydride batteries, and human beings have only developed and utilized 20% to 30% of its theoretical power, so the development prospect is very bright. At the same time, it is a real green battery that will not pollute the environment, and is currently the best battery that can be applied to electric vehicles.
According to different materials, lithium batteries as car battery types can be divided into lithium iron phosphate battery, lithium cobalt oxide battery, and ternary lithium battery. As far as the actual loading situation on the market is concerned, the mainstream new models basically use ternary lithium batteries and lithium iron phosphate batteries. The following focuses on comparing the characteristics, advantages and disadvantages of these two batteries.
| Ternary lithium battery | Lithium iron phosphate battery | |
| Composition material | Li(NiCoMn)O2 (Li(NiCoAl)O2 | LiFePO4 |
| Energy Density | High (about 240WH/kg) | Low (about 140WH/kg) |
| Volume / Weight | Small/Light | Large/Heavy |
| Temperature resistance | Low temperature resistant, not high temperature resistant | High temperature resistant, not low temperature resistant |
| Low temperature resistance: The lower limit of low temperature use is minus 30 degrees, and the power attenuation is about 15% in winter | Not resistant to low temperature: The lower limit of low temperature use is minus 20 degrees, and the power is attenuated by about 30% in winter | |
| Not resistant to high temperature: The thermal runaway temperature is about 200°C~300°C, and the risk of spontaneous combustion is high | High temperature resistance: thermal runaway temperature above 500°C, low risk of spontaneous combustion | |
| Safety | Poor | Better |
| Cost | High | Low |
| Lifespan | Low cycle life (Charge and discharge starts to decay after about 2000 times) | High cycle life (Charge and discharge about 3500 times and start to decay) |
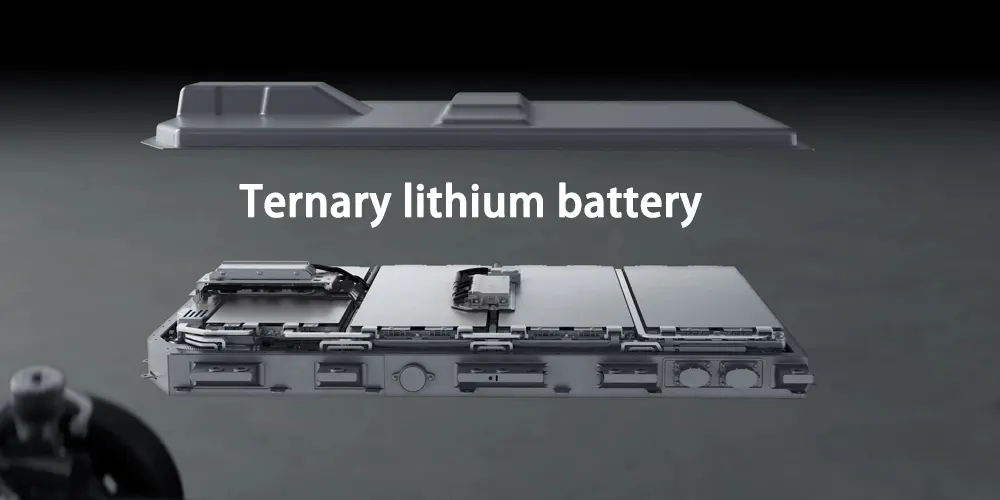
Ternary lithium battery
Ternary lithium battery refers to a battery that uses lithium nickel cobalt manganese oxide (Li(NiCoMn)O2) or lithium nickel cobalt aluminum (Li(NiCoAl)O2) ternary cathode material as the cathode material. Currently, the former is commonly used in electric vehicles.
The ternary composite cathode material is made of nickel salt, cobalt salt, and manganese salt. The ratio of nickel, cobalt and manganese in it can be adjusted according to actual needs. The battery with ternary material as cathode is safer than lithium cobalt oxide battery.
Among them, the advantages of ternary lithium batteries lie in energy storage density and low temperature resistance. First of all, in terms of energy storage density, because of the high voltage of the ternary lithium battery, its energy density can basically reach 240WH/kg, which is almost 1.7 times that of the lithium iron phosphate battery at 140WH/kg.
At the same time, in terms of the comparison between NCA and NCM batteries, NCA has better performance, but because of the lower thermal runaway temperature, it requires high manufacturing process and high cost, and the technology is in the hands of Japanese and Korean car companies.
Therefore, China mainly develops NCM batteries in car battery types, which are currently divided into four types according to the proportion of ternary materials: 111, 523, 622 and 811. Among them, 881 high-nickel batteries have become the key breakthrough direction because it is the key to improving the energy
The second is low temperature resistance. The lower limit of low temperature use of ternary lithium batteries is minus 30 degrees, which is more advantageous than the lower limit of minus 20 degrees for lithium iron phosphate batteries. Compared with lithium cobalt oxide batteries, ternary lithium batteries are safer and more suitable for the development trend of electric vehicle batteries in the future.
Lithium iron phosphate battery
Lithium iron phosphate battery is a lithium ion battery using LiFePO4 as the cathode material. Lithium iron phosphate has advantages in three aspects:
One is high safety, because the thermal runaway temperature of lithium iron phosphate batteries is generally higher than that of ternary lithium batteries. In contrast, lithium iron phosphate batteries have a lower risk of spontaneous combustion during high-speed driving and fast charging. In these car battery types, the stability of lithium iron phosphate battery is currently the best among lithium batteries for vehicles.
The second is longer cycle life, because lithium iron phosphate batteries will not start to decay until the number of charge and discharge cycles is greater than 3,500, which means that their service life can be as long as ten years or so. However, compared lfp vs nmc battery, the number of charge and discharge cycles of the ternary lithium battery is only 2,000, which means that its service life is only 6 years, which shows the gap between the two.
The third is lower manufacturing costs, because lithium iron phosphate batteries do not have precious metals, so production costs are lower. On the other hand, because ternary lithium batteries use cobalt metal, 70% of their reserves are in the Democratic Republic of Congo, Africa, which makes their import prices soaring all the way. This is another key reason why battery companies are forced to take the 811 technology route.
Fuel cells
A fuel cell is a chemical device that directly converts the chemical energy of fuel into electrical energy, also known as an electrochemical generator. Different from other car battery types, it converts the chemical energy stored in fuel and oxidant directly into electrical energy according to the electrochemical principle. The actual process is the redox reaction. The reaction product has no pollution to the environment.
Fuel cells are mainly composed of three parts, electrodes, electrolytes and external circuits. The fuel is mainly hydrocarbons such as hydrogen and methanol. Commonly used fuel cells can be divided into proton PEMFC, SOFC, MCFC, PAFC and AFC according to their different electrolytes.
Among them, PEMFC is a relatively mature and widely used fuel cell due to its various performance advantages, including low battery operating temperature and fast start-up speed, and occupies a dominant position in global shipments.
Supercapacitors
Supercapacitors, also known as farad capacitors, are electrochemical components developed in the 1970s and 1980s to store energy through polarized electrolytes. It is a power source with special performance between traditional capacitors and batteries. It uses the electric double layer structure composed of activated carbon porous electrodes and electrolytes to obtain super large capacity, and relies on the electric double layer and redox charges to store electrical energy.
There is no chemical reaction in the process of its energy storage, and this energy storage process is reversible, because this supercapacitor can be repeatedly charged and discharged hundreds of thousands of times.
The outstanding advantages of supercapacitors are high power density, short charge and discharge time, long cycle life, and wide operating temperature range. Compared with traditional car battery types products, supercapacitors can store only a small amount of electricity. Therefore, on a plug-in electric vehicle at home, this capacitor cannot be used alone as a power supply device, but must be used in conjunction with a fuel power battery.
On the other hand, supercapacitors have the ability to quickly discharge the stored electric energy. In addition, the charging time of this kind of capacitor is also very short, it only takes a few seconds or a few minutes to complete the charging, and the performance will not be reduced after multiple charging.