Home » lithium ion battery knowledge » Charging and discharging of lithium ion battery – principle and characteristics
Charging and discharging of lithium ion battery - principle and characteristics
Characteristics of lithium
Before getting to the charging and discharging of lithium ion battery, it is necessary to understand the structure of lithium ion batteries and the characteristics of lithium.
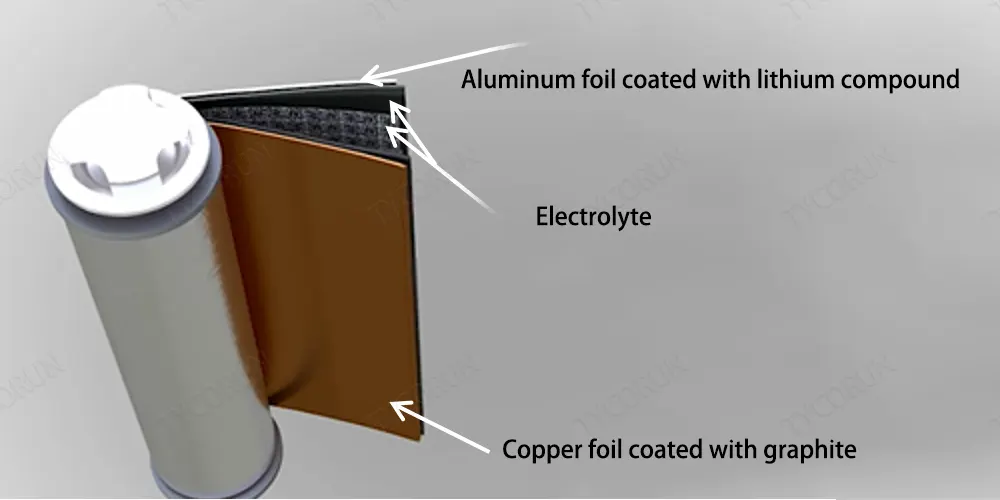
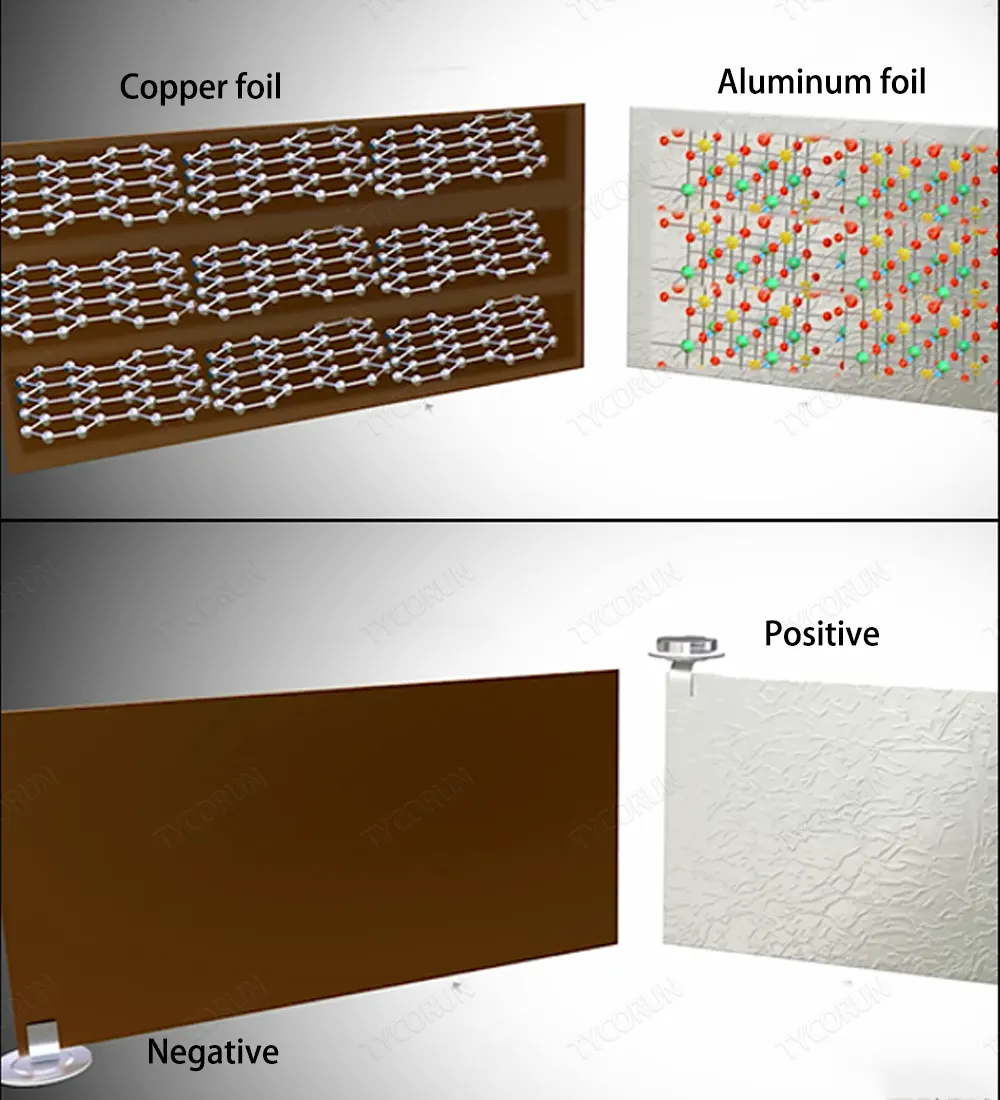
Lithium ion batteries consist of three parts, copper foil coated with graphite, aluminum foil coated with lithium compounds, and electrolyte containing organic salts of lithium. The charging and discharging of lithium ion battery is actually the reciprocating movement of lithium ions and free electrons.
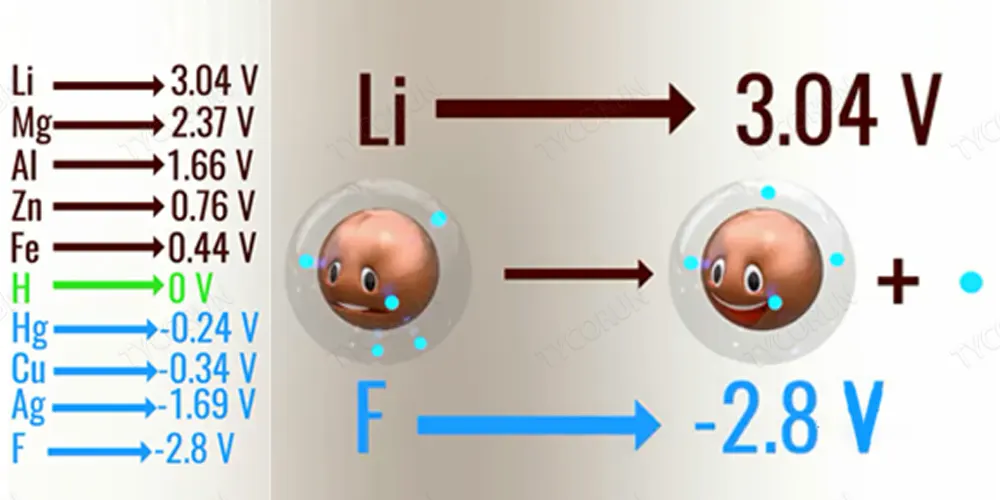
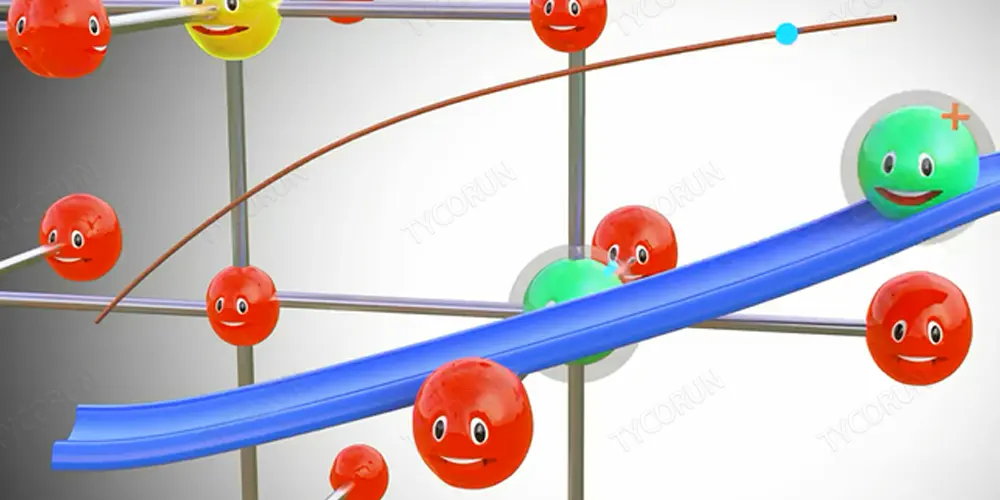
Different metals have different electrochemical potentials. Electrochemical potential is the tendency of metals to lose electrons. The electrochemical potentials of some common metals are shown in the figure below. Among them, lithium, which is most likely to lose electrons, is used in lithium-ion batteries.
As shown, lithium has the highest propensity to lose electrons, while fluorine has the least propensity to lose electrons. This is because the outermost layer of lithium has only one electron, which is very easy to lose; while the outermost layer of fluorine has 8 electrons, which is very stable (the outermost layer of the atom can only have 8 electrons at most, 1 is the most active, 8 is the most stable).
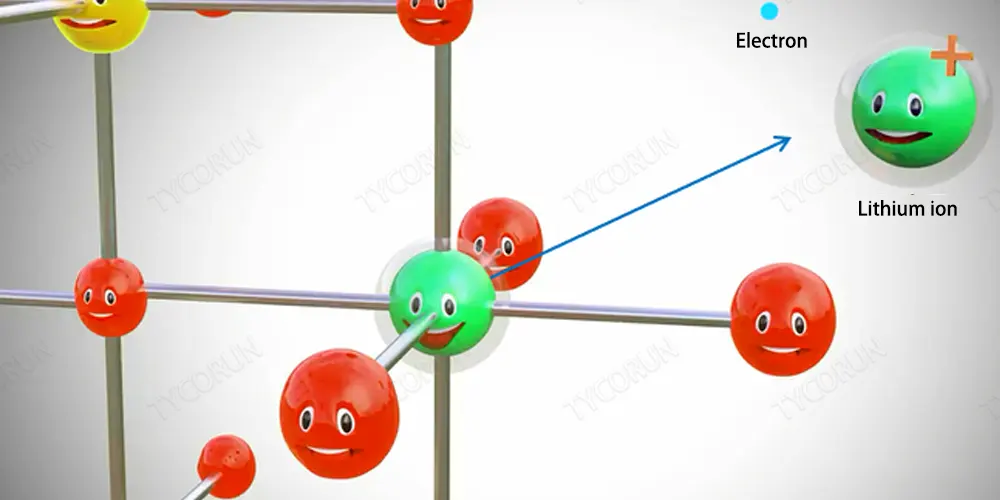
Pure lithium is a highly reactive metal that boils directly in water, but lithium metal oxides are very stable, and if we somehow separate a lithium atom from this metal oxide, the lithium atom is extremely unstable, will instantly form a lithium ion and an electron, as shown in the figure below.
However, as mentioned above, lithium, as a part of the metal oxide, is much more stable in the metal oxide than in the free state of lithium atom. Therefore, if two different paths can be provided for the flow of electrons and lithium ions between lithium and metal oxide, lithium atoms will automatically go to the metal oxide part, and the path of electron is the external circuit.
So when the electric field force is used to lift lithium out of the metal oxide, and then provide different paths for its ions and electrons (withdrawal of the external electric field), lithium will return to the oxide, as shown in the figure below.
Characteristics of lithium ion battery
Lithium ion battery is one of the batteries of highest energy density, delivering higher voltage and higher current per cell without the need for trickle charging when the battery is fully charged.
But a lithium ion battery has no memory effect, meaning it doesn’t “remember” how much power it has left until it’s completely drained, so a lithium ion battery must be charged using a special constant-current-constant-voltage (CC-CV) charging profile, and the charging curve can be automatically adjusted according to the battery temperature and voltage level.
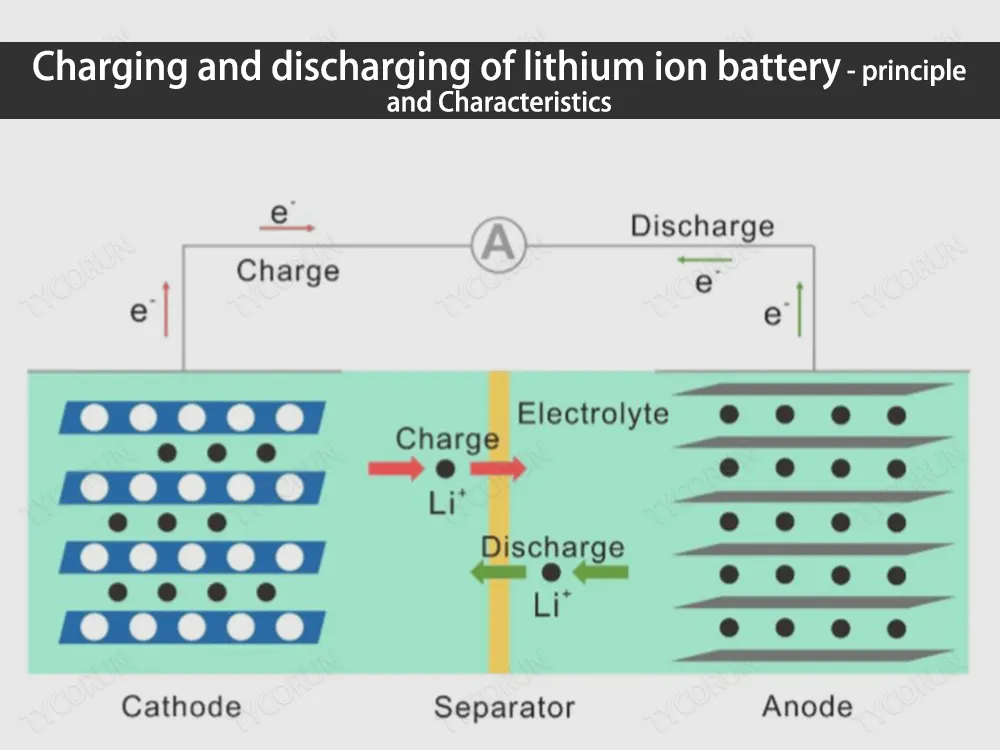
Charging and discharging principle of lithium ion battery
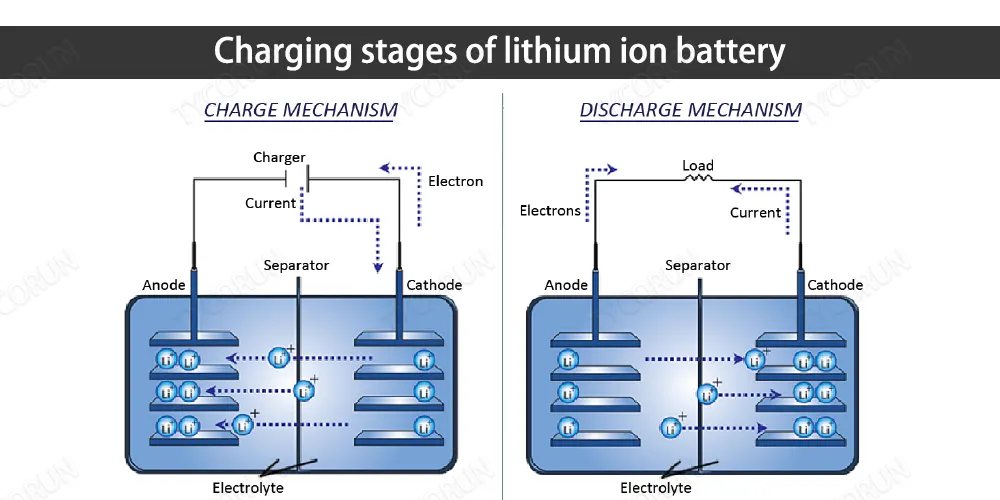
Lithium ion batteries contain electrolyte and graphite, which has a layered structure so that separated lithium ions can be easily stored there. The electrolyte between the graphite and the metal oxide acts as a protection, allowing only lithium ions to pass through, but not electrons.
When an external power source (electric field) is applied, due to the attraction of opposites, the cathode of the power source will attract electrons from the lithium atoms of the metal oxide, and push these electrons to flow along the external circuit and reach the graphite layer, while these electrons cannot flow through the electrolyte .
At the same time, positively charged lithium ions are also attracted to the anode and flow through the electrolyte to reach the graphite layer. At this time, the graphite layer contains a large amount of lithium ions and electrons. Among them, these ions and electrons are lithium atoms, but because lithium atoms are unstable, they exist in the form of lithium ions and electrons.
Once all the lithium atoms in the metal oxide reach the graphite layer, the battery is fully charged. At this time, the unstable state of the lithium atom is like a small ball on the top of the mountain. Once the power source is removed and the load is connected, the lithium atom will return to a stable state as a part of the metal oxide.
Because of this tendency to stabilize, the electrons are loaded back to the oxide, and the lithium ions travel through the electrolyte back to the oxide, like a ball sliding down a hill, and finally the lithium ions and electrons combine to form lithium atoms fixed in its metal oxide.
Throughout the process, graphite does not play a role in the chemistry of lithium-ion batteries, it is just a storage medium for lithium ions. If the internal temperature of the battery rises due to some abnormal situation and the electrolyte dries up, the lithium ions and electrons will all run to the oxide along the same path at this time, which causes a short circuit between the anode and the cathode, and may cause a fire or explosion.
To avoid this, an insulating layer called separator is placed between the electrodes. Due to its micropores, the separator is permeable to lithium ions but not electrons.
In the actual lithium battery, graphite and metal oxide are coated on copper foil and aluminum foil respectively, the foil here acts as a current collector, and the positive and negative plates can be easily removed from the collector, as shown in the figure below. Among them, the copper foil leads to the anode, and the aluminum foil leads to the cathode.
An organic salt of lithium is used as the electrolyte and is coated on the separator sheets, all of which are wound on a cylinder around a central steel core, making lithium cells more compact. A standard lithium ion battery has a voltage between 3v and 4.2v.
The charging and discharging of lithium ion battery is actually the reciprocating motion process of lithium ions and electrons. When charging, apply power to the battery to let lithium ions and electrons go to the graphite layer along different paths.
At this time, lithium atoms It is very unstable. And discharging is to apply a load to the battery, allowing lithium ions and electrons to run to the side of the metal oxide along the previous path. At this time, lithium atoms are relatively stable as a part of the metal oxide.
Charging stages of lithium ion battery
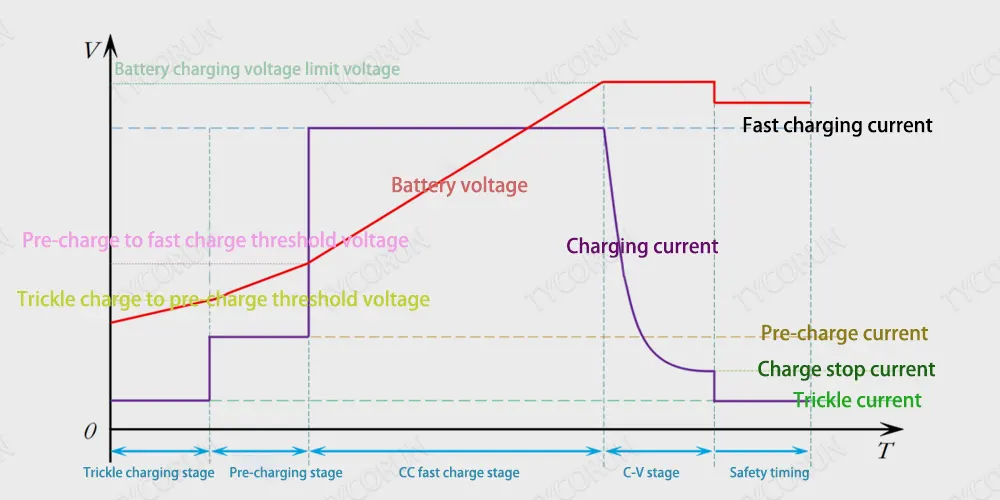
Stage 1. Trickle charge
If the battery voltage is lower than VBATT_TC (trickle charge pre-charge voltage threshold) (2V/cell), the IC will charge the battery with a trickle charge current of 100mA (adjustable). The trickle charge stage is usually only used when the battery voltage is below a very low level (about 2.1V). In this state, the battery pack’s internal protection IC may have disconnected the battery due to deep discharge or an overcurrent event.
The battery charger IC provides a small current (typically 50mA) to charge the battery pack’s capacitors to trigger the protection IC, which turns on its MOSFET to reconnect the battery. Although trickle charging usually lasts only a few seconds, the charging IC still needs to integrate a timer. If the battery pack is not reconnected within a certain amount of time, indicating that the battery is damaged, the timer stops charging.
Stage 2. Pre-Charge
Once the battery pack is reconnected or in a discharged state, it enters the pre-charge stage. During precharge, the charger IC begins to safely charge the depleted battery at a lower current level, typically C/10 (C is capacity in mAh). Pre-charging slowly increases the battery voltage, the purpose of which is to safely charge the battery at a low current level to prevent damage to the battery until its voltage reaches a higher level.
Stage 3. CC (Constant Current Charging)
CC charging is also known as the fast charging stage. Constant current charging starts after pre-charging and starts once the battery voltage reaches about 3v per cell (adjustable). During the constant current charging stage, the battery can safely output a higher charging current between 0.5C and 3C. Constant current charging continues until the battery voltage reaches a “full” or float voltage level, and then enters the constant voltage charging stage.
Stage 4. CV (Constant Voltage Charging)
The constant voltage (CV) threshold for lithium batteries is typically 4.1v to 4.5v per cell. The charging IC monitors the battery voltage during constant current charging. Once the battery reaches the constant voltage charging threshold, the charger IC transitions from constant-current to constant-voltage regulation.
When the charger IC detects that the voltage of the external battery pack exceeds the actual battery voltage in the battery pack, it starts to perform constant voltage charging.
This is due to the presence of internal battery resistance, PCB resistance, and equivalent series resistance (ESR) from the MOSFETs and cells on the protection board PCM. The charging IC does not allow the battery voltage to exceed its maximum float voltage to ensure safe operation of the charging system.
Stage 5. Stop charging
During the constant voltage charging stage, when the current flowing into the battery drops below the set threshold (approximately C/10), the charger IC terminates the charging cycle. At this point the battery is considered fully charged and charging is complete. If the charge cutoff function of the charger IC is disabled, the charge current will naturally decay to 0mA, but this is rarely done in practice.
Because the amount of charge going into the battery drops exponentially during constant voltage charging (the battery voltage increases like a big capacitor), it takes a very long time to charge the battery with very little increase in capacity.
C rate of lithium ion battery
C is for capacity, the abbreviation of capacity, and the “C rate” of the battery specifies the maximum current for charging and discharging of lithium ion battery. Standard C rates are typically between 0.5C and 3C, depending on the specific cell used, and there is often a trade-off between higher C rates and lower energy densities.
For example, for a 3000mAh battery, the C rate is 1C, which means the battery can be charged with a maximum current of 3A. Typically, battery manufacturers also specify different voltage and temperature ranges for the C rate, with the C rate being reduced at lower voltages or higher/lower temperatures.
If a battery has a higher C rate, we call it a high rate battery, and it can release more current and charge faster. For example, batteries with higher C rates are more useful for portable devices such as smartphones and laptops than wireless speakers, because these portable devices may need to be charged at least once a day.
Usually fast charging is definitely the first choice for devices that run for a short time and need to be used continuously. Knowing the C rate of a battery can help lithium ion battery company determine the charging current, and optimize their charging profile solution, and choose the best charger IC topology and safety features for the battery.