Lithium ion battery cathode material industry report - spinel nickel manganate lithium

Lithium ion battery cathode material
Determining factors of energy density of lithium batteries
It can be seen that higher lithium ion battery cathode specific capacity, higher anode specific capacity and higher battery voltage (and fewer auxiliary components) are the theoretical realization paths for high energy density batteries.
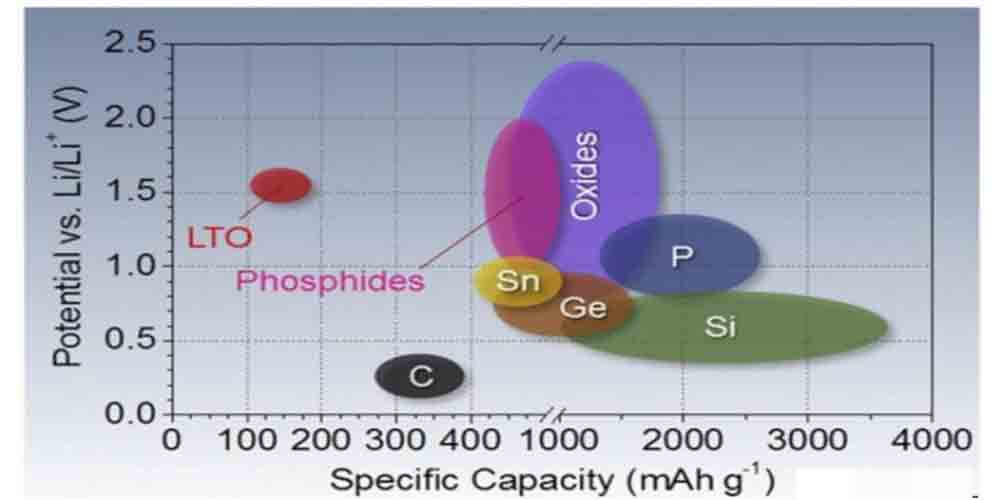
According to the basic principles of lithium storage, both cathode and anode materials can be divided into two categories: phase change materials and intercalation materials.
There are four types of materials in terms of capacity and voltage range against lithium: intercalation type materials generally have lower capacity, while phase-change materials have higher capacity; lithium ion battery cathode materials have lower capacity, and negative electrode materials have higher capacity.
Typical lithium ion battery cathode material specific capacity and voltage to lithium
The main body of cathode and anode materials currently used in large-scale applications is intercalation type materials. Some phase-change anode materials, represented by silicon, have obtained a small amount of practical applications through doping.
The phase-change lithium ion battery cathode materials, including chlorides, sulfides, fluorides, iodides, etc., although scientific research efforts have continued, are limited by material kinetics factors, comprehensive performance trade-off limitations, etc., and the maturity of practical applications is still low.
The high-quality and low-cost graphite negative electrode can achieve a capacity close to 370mAh/g, not to mention the silicon-based negative electrode; while the capacity of the lithium ion battery cathode system with relatively high relative voltage (over 3V on average) is still within 300mAh/g. This also makes the problem of insufficient lithium ion battery cathode capacity in the active material system of the entire lithium battery, which affects the overall performance of the battery, particularly prominent.
Bottlenecks have the highest status. Another obvious example that reflects the importance of lithium ion battery cathodes is that Mr. Goodenough ranks first among the three Nobel Prize winners in the field of lithium batteries for his invention of various lithium ion battery cathodes.
Lithium ion battery cathode structure for commercial application
Layered materials, spinel and olivine, lithium ion battery cathode structures for commercial applications. The currently widely used intercalation lithium ion battery cathode materials include three systems of layered materials, spinel and olivine according to different crystal structures.
The layered lithium ion battery cathode material represents, for example, lithium cobalt oxide and ternary materials; the spinel lithium ion battery cathode material represents, for example, lithium manganate; and the olivine lithium ion battery cathode material represents, for example, lithium iron phosphate.
The two technical routes of iron-lithium and ternary have been in a stalemate for a long time. Related industry information can be learned from Top 5 lithium iron phosphate cathode material companies and Top 5 cathode ternary material companies. High nickel and high voltage are the technical trends for improving the performance of layered lithium ion battery cathode batteries.
The march from iron lithium to iron manganese lithium is a relatively feasible method for olivine structure lithium ion battery cathode batteries to supplement the short board of energy density.
For the spinel structure lithium ion battery cathode, efforts to improve the corresponding battery energy density and optimize the overall performance have not stopped.
Typical anode material specific capacity and voltage to lithium
Lithium Nickel Manganate
Lithium nickel manganate, spinel high-voltage lithium ion battery cathode derived from lithium manganate.
Spinel structure lithium ion battery cathode
Among the different technical types of lithium ion battery cathode, lithium manganate was the first to be invented.
The elements of lithium manganate material are cheap and easy to obtain; its crystal structure corresponds to a three-dimensional diffusion channel, the volume change caused by lithium ion intercalation and deintercalation is relatively small, and the material rate performance is good.
However, some +3-valent manganese ions (which have been shown to be easily enriched on the surface of lithium ion battery cathode particles) will seriously distort the manganese-oxygen octahedron (the so-called J-T effect), causing material cracking and aggravating electrode-electrolyte side reactions;
The +3-valent manganese ions will also undergo disproportionation and dissolution. The manganese dissolved in the electrolyte and finally deposited on the negative electrode will accelerate the decomposition of the electrolyte, thicken the SEI film on the surface of the negative electrode, and consume the active lithium in the system; lithium manganate lattice It is also easy to carry/form some oxygen vacancies in it, aggravating the performance deterioration.
Side reactions are exacerbated at high temperatures. All these reasons make the life of the battery corresponding to the lithium manganate lithium ion battery cathode relatively low, not as good as the battery corresponding to the lithium iron and ternary lithium ion battery cathode.
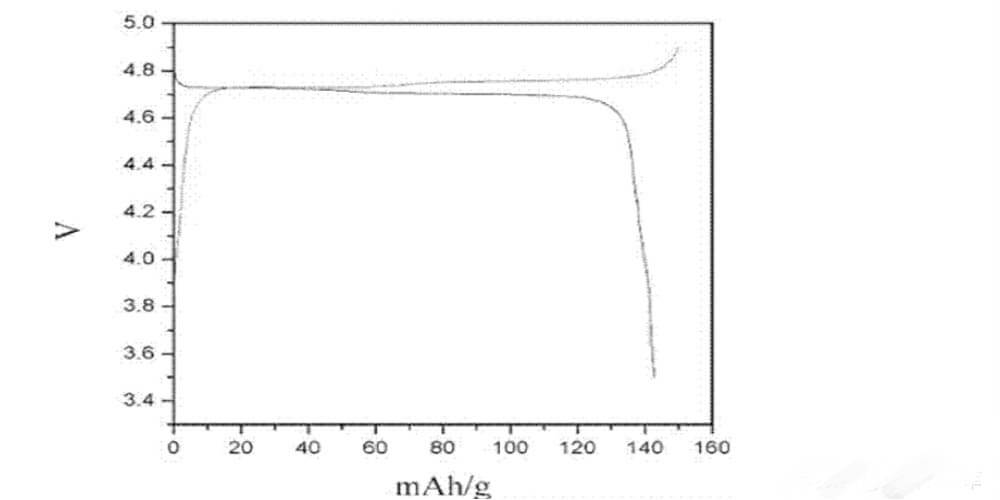
In addition, the capacity and voltage performance of the lithium manganate cathode are also unsatisfactory. The upper limit of the specific capacity is only about 148mAh/g*, which is lower than the upper limit of the specific capacity of the olivine structure lithium ion battery cathode, which is 170mAh/g, and far lower than the upper limit of the specific capacity of the layered structure lithium ion battery cathode, which is 274mAh/g;
The average voltage to lithium is 4V, and the average specific capacity is only about 115mAh/g under the average voltage to lithium. This makes the system energy density of lithium manganate cathode battery low.
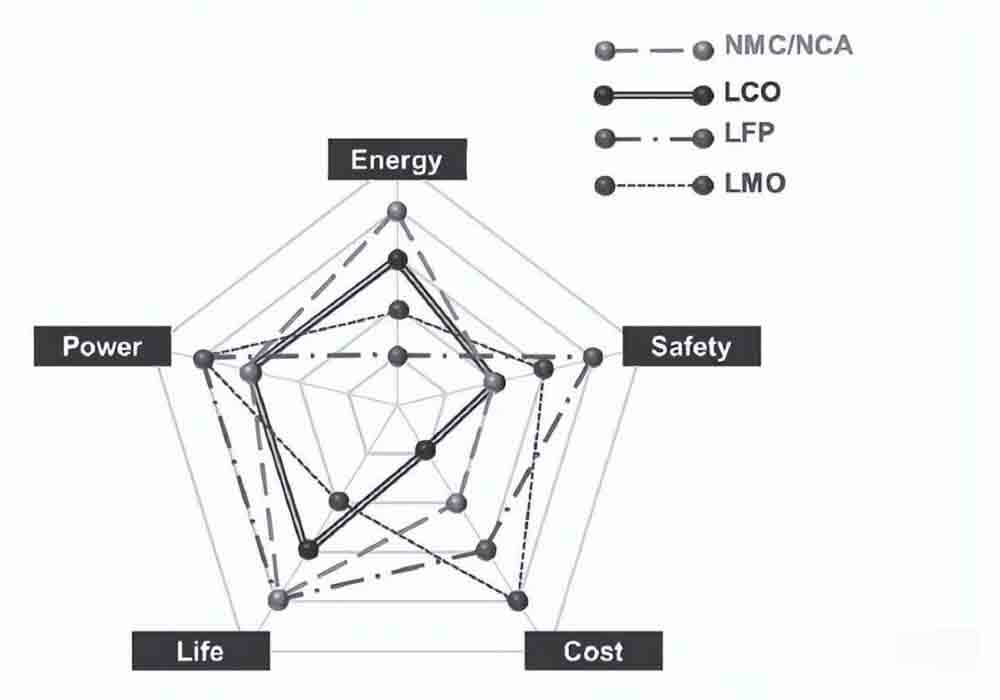
Comprehensive performance of different lithium ion battery cathode materials corresponding to lithium batteries
Taking the lithium manganate cathode as an example, this paper also introduces the theoretical specific capacity calculation method of the electrode material:
1. Give the material chemical formulas of the theoretical lithium intercalation state and the theoretical delithiation state, which are LiMn2O4 and Mn2O4 respectively for lithium manganate;
2. Calculate the relative molecular mass of the material, lithium manganate is 180.81;
3. Calculate the percentage of the material changed by lithium corresponding to the intercalation state and delithiation state of the material, and lithium manganate is 100%;
4. Taking 1 mol of this material as a comparison benchmark, to calculate the amount of charge transferred by lithium, it is necessary to use the Faraday constant of 96485C/mol and the conversion relationship of C-Ah 3600 times;
5. Divide the final electricity consumption by the relative molecular mass to obtain the theoretical specific capacity, and the unit is unified as mAh/g. The calculated result of the lithium manganate cathode is 26.80Ah/180.81g=148mAh/g.
Similarly, it can be calculated that the theoretical capacity of lithium iron phosphate is 170mAh/g, and the theoretical capacity of ternary lithium is 274mAh/g.
Taking the industry standard conditions of lithium-ion batteries issued by the Ministry of Industry and Information Technology as a reference, the specific capacity of lithium manganate reaches 115mAh/g, which is equivalent to 77% of the theoretical specific capacity; the specific capacity of iron and lithium reaches 145mAh/g, which is equivalent to 85% of the theoretical specific capacity;
The specific capacity of the ternary material reaches 165mAh/g, which is equivalent to 60% of the theoretical specific capacity. The actual specific capacity is related to the theoretical specific capacity, as well as the boundary conditions of lithium intercalation/deintercalation, etc.)
The result of a comprehensive balance is that lithium manganate is only suitable for applications that do not require high life and energy density and are very cost-sensitive, such as two-wheeled electric vehicles;
The optimistic expectation is that there will be a slight scale application in some low-speed electric vehicles and A00-class models.
There are many ways to modify the lithium manganate cathode. Under the condition that lithium manganate matrix is still used, various doping and coating methods have been studied, such as a small amount of aluminum doping to replace manganese to improve cycle life, titanium dioxide nanobelt doping to optimize effective capacity and so on.
However, if you want to greatly improve the overall performance of the battery corresponding to the lithium manganate lithium ion battery cathode , the basic idea is also: the energy density must be effectively improved.
Because the theoretical upper limit of the specific capacity of lithium manganate is limited, it is necessary to build a new material matrix to greatly increase the voltage to lithium, and to simultaneously optimize the bottleneck factors such as the comprehensive performance of the battery, so that it can meet the needs of electric vehicles.
Aluminum-doped lithium manganate improves life performance
Under the guidance of this idea, the related research on high-voltage lithium nickel manganate cathode materials has gradually become the winner of the spinel structure cathode.
Research progress of lithium nickel manganate substrate
After uniformly replacing 25% of manganese with nickel, so that LiMn2O4 becomes lithium nickel manganate-LiNi0.5Mn1.5O4, the lithium ion battery cathode material obtained by the researchers has an upper limit of lithium-to-lithium voltage as high as 5V, and a voltage platform of about 4.7V, which makes The energy density of the battery cell can be directly increased by about 20% (to the level close to the energy density of the ternary battery);
In principle, the valence of manganese in lithium nickel manganate is +4, which makes it less affected by lattice distortion than conventional lithium manganate with a considerable amount of manganese at +3, and the overall performance is improved.
But at the same time, we must also note that although theoretically, the valence of nickel in lithium nickel manganate is +2 and the valence of manganese is +4, but in fact, some oxygen vacancies are still generated during the synthesis process (high temperature synthesis is obvious) and the manganese is reduced to + 3 valence, which makes lithium nickel manganate not completely free from the effect of lattice distortion.
In addition, higher voltages also pose serious challenges to existing electrolyte systems, and conventional carbonate components face the danger of decomposition;
Lithium nickel manganate is not resistant to corrosion by hydrofluoric acid (a trace amount of water in the electrolyte will decompose 6F to generate hydrofluoric acid), and manganese will still be dissolved after being corroded. This makes the practical application of lithium nickel manganate requires effective synthesis and modification methods.
The synthesis methods of lithium iron manganese phosphate include traditional solid-phase method, precursor precipitation-solid-phase calcination method, sol-gel method, and relatively special molten salt method using low melting point salt melt.
The traditional solid-phase synthesis method is to directly mix manganese sources such as manganese carbonate, manganese trioxide, manganese tetroxide, manganese dioxide and nickel sources, lithium sources and calcining;
The synthesis route of the precursor precipitation-solid phase calcination method is similar to the principle of ternary material synthesis. The precursor precipitation is prepared first, and then the synthesis reaction is carried out. Generally speaking, the solid-phase method is relatively convenient, but high-temperature calcination causes many oxygen vacancies and impurities.
Titanium dioxide nanoribbons doped with lithium manganate improve capacity performance
The basic process of the sol-gel method is to prepare a sol containing lithium, nickel and manganese, drying and calcining to form a lithium nickel manganate lithium ion battery cathode . The advantage is that the particle has a high degree of crystallization and good dispersibility; the disadvantage is that the cost is high and the reaction speed is relatively slow.
The size effect of lithium nickel manganate shows that the capacity-voltage performance of 300 nm particles is better than that of 1 micron particles at different rates. Of course, uniform particles may have a negative effect on compaction density to some extent; larger specific surface areas may also induce more side reactions.
Bulk element doping is one of the main methods for the modification of lithium nickel manganate. The main purpose to be realized is to expand the solid solution region of the material, improve the rate performance, and improve the structural stability and thermal stability.
Cationic elements such as aluminum, chromium, copper, zirconium, magnesium, cobalt, titanium, and anionic elements such as fluorine, chlorine, phosphorus, sulfur, etc., all received relatively positive evaluations.
Surface coating is another method to improve the performance of lithium nickel manganate. The main purpose is to inhibit the dissolution of manganese and the side reactions of electrodes and electrolytes, so as to improve the first effect and improve the cycle life.
For example, a simple carbon material coating can improve the battery cycle life, and some oxides, organic substances, etc. also have positive effects. In addition, some other surface treatment methods can also play a similar role.
Influence of different modification methods on the capacity performance of lithium nickel manganate cathode. After doping, coating, grain morphology and size control, the capacity of LiMnO2 lithium ion battery cathode can be close to its theoretical capacity.
Electrolyte modification corresponding to lithium nickel manganate
The high voltage continues to decompose the traditional electrolyte, and the trace amount of hydrogen fluoride erodes the lithium nickel manganate cathode, which together affect the performance and life performance of the lithium nickel manganate battery.
The aforementioned surface coating of the lithium ion battery cathode material is one of the methods to solve the problem, but it is also inevitable to face the problems of affecting the discharge capacity and complicating the production process of the lithium ion battery cathode material.
The effect of carbon coating on the rate performance of LiMnO4 (a Uncoated)
In addition to modifying the lithium ion battery cathode material, adjusting the components of the electrolyte system can also play a role in improving battery performance
(Or directly withstand voltage, or some components decompose on the surface of the lithium ion battery cathode to form a CEI protective layer). This work relies on lithium salts, solvents and other additives.
Studies have shown that using 6F alone as a lithium salt, compared with using 6F and LiBOB at the same time, lithium dioxalatoborate can passivate the surface of the lithium ion battery cathode and improve the cycle life of the battery.
Moreover, the dioxalatoborate in LiBOB can consume the trace amount of hydrogen fluoride in the system, and generate a small amount of lithium difluorooxalateborate LiDFOB and lithium tetrafluoroborate during the battery cycle, which is beneficial to the overall performance of the battery.
There is also research work showing that the electrolyte system of LiFSI-ethylene glycol dimethyl ether plays a significant role in the cycle performance of spinel lithium nickel manganate.
Fluorinated carbonate solvents have a higher oxidation potential and are used to replace conventional carbonate solvents, which can improve the life performance of lithium nickel manganate batteries at room temperature and high temperature.
Studies have shown that the use of fluorinated ester solvents reduces the decomposition products of both the lithium ion battery cathode and the negative electrode in the material system, which means that the stability of the entire battery system has been improved.
For example, fluoroethylene carbonate FEC can effectively improve the charge-discharge performance of lithium nickel manganate-silicon-based lithium batteries.
In addition, nitrile and sulfone solvents have relatively good pressure resistance; various additives containing phosphorus, boron, sulfur, and silicon are also used to improve the performance of lithium nickel manganate batteries; some ionic liquids also have similar effects ; Concentrated salt can also improve the pressure resistance of the electrolyte to a certain extent.
Finally, solid electrolytes may also be an alternative electrolyte for high-performance LiMnO2 batteries.
Performance characteristics of different electrolyte solvent systems
Of course, high-voltage electrolytes also have problems to varying degrees. Such as sulfone solvents and ionic liquids are viscous and have poor wettability;
Concentrated salt directly pushes up the cost; the problem of contact deterioration of the electrolyte-electrode interface of the solid electrolyte is not easy to solve, and so on.
Breakthrough in specific capacity
“Lithium-rich” lithium nickel manganate material
While making progress in high voltage, researchers have not given up their efforts to improve the specific capacity of the spinel phase lithium ion battery cathode, thereby increasing the energy density.
The theoretical specific capacity of the lithium nickel manganate cathode is only 148mAh/g, and the only way to break through the theoretical specific capacity is to build a “lithium-rich” lithium nickel manganate material system with high reversible capacity.
The academic literature Li2Ni0.5Mn1.5O4, spinel type lithium ion battery cathode material with high reversible capacity published in 2019 reported the research on the realization of a reversible specific capacity of 260.4mAh/g at a low rate of lithium-rich nickel manganate, which is close to the theoretical specific capacity of 282.6mAh/g results.
The research work adopts the traditional solid-phase method as the synthesis method: deionized water is mixed with nickel oxide, manganese carbonate and lithium carbonate in the proportion of substances, the suspension is ball-milled, dried and calcined in air in multiple stages.
The average particle size of the obtained lithium-rich nickel lithium manganate primary particles is about 300 nanometers; the average particle size of the secondary particles is about 3 microns. The particle size distribution of solid-phase method particles is wider.
Further research shows that redox couples such as Mn2+/Mn3+, Mn3+/Mn4+, Ni2+/Ni3+, and Ni3+/Ni4+ appear successively during the material cycle.
Compared with the theoretical nickel divalent and manganese tetravalent lithium nickel manganese oxide, the proportion of trivalent manganese in the lithium-rich nickel manganese oxide is significantly increased to maintain the electrical neutrality of the material.
Under the condition of 0.05C rate cycle, the specific capacity of lithium-rich nickel manganate reaches 260.4mAh/g, the theoretical energy density of the battery (only the active material is considered, and cannot be directly compared with the actual energy density of the existing ternary and iron-lithium batteries). Comparison) reaches 890mAh/g, which exceeds 735mAh/g of high nickel ternary battery and 660mAh/g of lithium nickel manganate battery.
Capacity and energy performance of lithium-rich nickel manganese
On the other hand, the cycle life and rate performance of lithium-rich nickel manganate are average. Under the condition of 0.05C, the capacity decreased by 20% after 50 cycles; when the rate was increased to 1C, the effective capacity was seriously reduced to 100mAh/g (the capacity can be recovered by reducing the rate again).
The researchers concluded that the deterioration of cycle life and low rate performance may be caused by the dissolution of manganese and the poor pressure resistance of the electrolyte.
The smoothness of lithium intercalation and deintercalation due to the lithium-rich composition may also be one of the reasons; the wide grain distribution caused by the solid-phase synthesis method may also have a negative effect.
In addition, lithium-rich lithium manganese nickelate can be used as a competent “lithium supplement” for lithium nickel manganate to a certain extent. Moreover, the effective modification means for lithium nickel manganate may also have a positive effect on lithium rich nickel manganate. The research work in this area can only be considered as just beginning.
Patent layout of lithium nickel manganate
Patent scale of lithium nickel manganate
The number of patents with valid, substantive examination and disclosure status of lithium nickel manganate battery cathodes is relatively small (some patent titles give similar names to cathodes with balanced nickel and manganese content), and the main application volume is in China.
In recent years, the patent applications for lithium nickel manganate have generally shown an increasing trend (from 2019 to the present, some patents have not been published).
In terms of applicants, the main applicants for lithium iron manganese phosphate patents are universities and research institutes, as well as some battery and lithium ion battery cathode companies.
Typical patent content – synthesis and modification
Patent CN104364943B issued by LG in 2017 describes a high-voltage lithium ion battery cathode active material consisting of a high-voltage spinel matrix and a carbon material coating.
The substrate corresponding to the embodiment is lithium nickel manganate, which is mixed with petroleum-based asphalt and heat treated at 500 degrees to complete the coating modification. After coating, the main performance index Examples are ahead of the Comparative Examples to varying degrees.
LG also studied the effect of the solvent system on the performance of lithium nickel manganate batteries, and the solvent system of 10% ethylene carbonate and 90% propyl formate had the greatest positive effect on battery performance. See patent US10170796B2.
The patent CN109560259A published by CATL in 2019 describes a method for coating a lithium iron phosphate-carbon layer on the surface of spinel phase lithium nickel manganate.
The researchers believe that the coating structure containing lithium iron phosphate and carbon can increase the conductivity of lithium ions and electrons at the same time, which can effectively increase the gram capacity and the first Coulomb efficiency of the lithium ion battery cathode material, and can effectively alleviate the flatulence problem of lithium nickel manganate cells.
From the examples, the researchers first mixed and sintered the lithium source, nickel source, manganese source, and doped metal source of spinel lithium nickel manganate to form a spinel phase; source, iron source, carbon source and lithium nickel manganate are mixed and granulated to obtain a precursor; and then sintered to obtain the desired lithium ion battery cathode material.
The described lithium iron phosphate coating layer can be prepared by the reaction of iron phosphate and lithium carbonate, or by the reaction of lithium hydroxide, ferrous oxalate and ammonium dihydrogen phosphate.
After coating, the gram capacity (over 130mAh/g) and the first effect (over 90%) of the examples are better than those of the comparative examples to varying degrees; the gas production in the 60-degree thermostatic box is compared, and the examples are also significantly better than the comparison examples. Proportion.
The patent CN109817968B issued in 2021 describes a method for coating lithium vanadyl phosphate on the surface of spinel phase lithium nickel manganate.
The researchers believe that the coating of lithium vanadyl phosphate can prevent the direct contact between lithium nickel manganate and the electrolyte, alleviate the disproportionation reaction of Mn3+ and the oxidation of Mn4+, relieve the flatulence problem of the cell, and improve the battery capacity and the first coulombic efficiency.
Improved gas production of lithium nickel manganate coated with iron lithium-carbon layer
From the examples, for the synthesis of lithium nickel manganate, nickel manganese oxide and nickel manganese hydroxide can be used as transition metal sources; lithium carbonate, lithium hydroxide and lithium nitrate can be used as lithium sources.
For the coating layer of lithium vanadyl phosphate, vanadium pentoxide, ammonium vanadate, etc. can be used as the vanadium source, and ammonium dihydrogen phosphate, etc., as the phosphorus source. After coating, the gram capacity (over 130mAh/g) and the first effect (over 90%) of the examples are better than those of the comparative examples to varying degrees; the gas production in a 60-degree incubator is compared, and the examples are also significantly better than the comparison examples. Proportion.
The patent CN102569776A published by Guoxuan Hi-Tech in 2012 describes a method for preparing a spherical high-voltage lithium ion battery cathode material spinel lithium nickel manganate by a hydrothermal-solid phase two-step method.
First, the nickel source, the manganese source and the doping element compound solution and the carbonate solution of sodium/ammonium are mixed uniformly, then a surfactant is added therein, and the spherical nickel-manganese carbonate coprecipitation is prepared under hydrothermal conditions;
After washing and drying, spherical nickel-manganese oxide is obtained by sintering; the oxide and lithium source are mixed by liquid-phase ball milling, dried, and finally sintered to obtain a lithium ion battery cathode active material. The researchers believe that the synthesis process of this method is simple, the process is easy to control, and the physical and chemical properties of the material can be optimized.
From the example, the cobalt-doped nickel-manganese carbonate precursor prepared by hydrothermal reaction at 120 degrees for 10 hours was ball-milled with lithium carbonate and calcined to form lithium nickel-manganese oxide. The discharge capacity, the voltage platform is about 4.7V; the 0.2C50 cycle capacity retention rate of the battery with conventional electrolyte and lithium metal is about 95%.
The patent CN103579610B, which was authorized in 2016, describes a method for preparing lithium nickel manganate by co-precipitation method and solid-phase sintering method using oxalate and hydroxide as precipitants at the same time.
The researchers believe that the solubility products of manganese oxalate and nickel hydroxide are similar. By controlling the pH value of the reaction solution, a uniform manganese oxalate/nickel hydroxide composite precursor material can be obtained.
After mixing with the lithium source, the lithium ion battery cathode material lithium nickel manganate is synthesized by sintering, which saves the step of using only hydroxide as the precipitant and requires inert gas protection, and reduces the production cost;
Compared with lithium nickel manganate prepared using only oxalate as precipitant, the tap density is effectively improved.
Hydrothermal-Solid-Phase Lithium Nickel Manganate Capacity-Voltage Performance
From the examples, the mixed precipitant using sodium oxalate-sodium hydroxide has a higher specific capacity than the sample using potassium oxalate-sodium hydroxide.
The patent CN104638259B, which was authorized in 2017, describes a method for modifying lithium nickel manganate cathode with lithium vanadate.
Firstly, the mixture of nickel source, manganese source and lithium source is calcined at low temperature to obtain the precursor; then the lithium source and vanadium source are mixed at high temperature and calcined to obtain the desired lithium ion battery cathode.
The modified nickel-lithium manganate material has small polarization and good cycling performance.
From the examples, a few percent lithium vanadate coating can effectively improve the battery cycle life at 2C rate.
Guoxuan Hi-Tech also studied the effect of other types of surface (coating) modifiers on the performance of lithium nickel manganate cathodes, such as cerium-iron composite oxides.
The patent CN109088067B authorized by Bangpu in 2020 describes the preparation method of low cobalt doped spinel-layered structure lithium nickel manganate two-phase composite lithium ion battery cathode material: using nickel salt and manganese salt to prepare spinel-structured nickel respectively The manganese precursor and the layered structure nickel-manganese precursor; the spinel-structured nickel-manganese precursor, the layered-structured nickel-manganese precursor, the lithium source and the cobalt source are uniformly mixed, and then calcined to obtain a composite lithium ion battery cathode material. The researchers believe that the spinel phase can improve the stability of the layered structure, and the layered structure has a low activation barrier for lithium ion migration, which can improve the rate performance. At the same time, cobalt doping can inhibit the mixing of lithium and nickel and improve the conductivity.
From the examples, the composite lithium ion battery cathode (mainly layered structure) was charged to 4.5V, the specific capacity was 175.6mAh/g, and the capacity retention rate was 98.8% after 80 cycles at room temperature and 2C rate.
The patent CN113707875A published by SVLOT in 2021 describes a method for metal oxide coating of spinel phase lithium nickel manganate containing doping elements. The researchers believe that doping and coating significantly improve the specific capacity, first effect and cycle performance of lithium nickel manganate.
The capacity voltage of lithium nickel manganate corresponding to sodium oxalate-sodium hydroxide precipitant
From the examples, the samples are doped with chromium oxide, zirconium oxide, titanium oxide, etc., and then coated with zirconium oxide. The first discharge capacity at 0.1C generally exceeds 130mAh/g, the first efficiency exceeds 90%, and the cycle retention rate at 1C50 generally exceeds 99%. %.
The patent CN113845152A published in 2021 describes the preparation of spinel lithium nickel manganate precursor by gel reaction, and the subsequent method for preparing the lithium ion battery cathode: lithium source, nickel source, manganese source, water, β-cyclodextrin, complexation The precursor gel is obtained by performing gel reaction with an agent and an alkaline regulator, and the precursor gel is dehydrated and calcined to obtain a lithium nickel manganate cathode material.
The researchers believe that the above preparation method can realize the anchoring of nickel and manganese, thereby reducing the probability of mixing lithium ions and nickel ions in the lithium nickel manganate cathode material, which is conducive to improving its structural stability and forming a spinel structure. The prepared lithium nickel manganate cathode material has good initial discharge efficiency, rate performance, capacity recovery and cycle stability.
From the embodiment, the lithium source includes lithium acetate, lithium ethoxide, etc., the nickel source and the manganese source are nickel acetate and manganese acetate, and the complexing agent is citric acid. The finally obtained lithium ion battery cathode (large single crystal particles) has an initial discharge capacity of more than 130mAh/g and an initial effect of more than 90%; 2C discharge capacity is usually above 130mAh/g; 10C discharge rate is close to 110mAh/g; 50-cycle capacity The retention rate generally exceeds 99%.
The patent CN113178566A published by Rongbai in 2021 describes a method for preparing a single crystal cobalt-free spinel nickel lithium manganate lithium ion battery cathode by co-precipitation and calcination.
The researchers described that the precursor was first prepared: under the action of a precipitant, ammonia water and a complexing agent, a nickel source and a manganese source were added to carry out a co-precipitation reaction to obtain a Ni0.5Mn1.5(OH)4 binary precursor; Then the lithium ion battery cathode synthesis is carried out: the precursor is mixed with the lithium source, calcined at a high temperature, then annealed at a low speed for heat preservation, and finally cooled naturally to obtain a single crystal LiNi0.5Mn1.5O4 cathode material.
Capacity-voltage curves of co-precipitated-calcined single-crystal spinel lithium nickel manganate cathodes, and corresponding battery cycle performance
From the embodiment, the transition metal central ion source can be selected from corresponding sulfates, acetates, etc.; the lithium source can be selected from lithium hydroxide, lithium carbonate and the like.
Single-crystal high-voltage lithium nickel manganese oxide material obtained by using manganese sulfate, nickel sulfate and lithium carbonate, the first effect is 94%, the 1C gram capacity is 135.2mAh/g, the 100-cycle capacity retention rate at 1C room temperature is 97.2%, and the 2C gram capacity is 97.2%. 128.2mAh/g, 3C gram capacity 126.3mAh/g.
The soft-pack full battery has a 1C gram capacity of 126mAh/g, and a 200-cycle capacity retention rate of 83% at 1C normal temperature.
Summary
Classical Inoculation Process of Inorganic Nonmetal Oxides
From the synthesis point of view, the spinel phase lithium nickel manganate can be obtained by various solid-phase methods, co-precipitation methods, sol-gel methods, and hydrothermal methods to obtain the desired phase, and can also be modified by metal element doping during the synthesis process. sex.
From the perspective of surface coating, the utilization rate of carbon and metal oxides is relatively high.
In addition, the spinel phase lithium nickel manganate can be combined with the iron-lithium lithium ion battery cathode (iron-lithium as the coating layer), and can be combined with the ternary lithium ion battery cathode (the two are doped with each other).
Estimation of energy density
Of course, as a high-voltage lithium ion battery cathode material, spinel phase lithium nickel manganese oxide also has research work on supporting voltage-resistant electrolyte, and the importance of supporting electrolyte system is very high.
Finally, in terms of battery performance, the energy density of the spinel phase lithium nickel manganate cathode may be close to that of the ternary lithium ion battery cathode.
Because the synthesis route has not yet been determined, and the composition and dosage of the negative electrode, electrolyte, separator and other components have not been determined, the cost of the spinel nickel lithium manganate lithium ion battery cathode and the corresponding battery cannot be quantitatively analyzed for the time being.
However, from the first principles, the expensive elements (lithium, cobalt, nickel) of lithium nickel manganate are used relatively little.
See here to learn more about lithium cobalt oxide cathode material companies, you can refer to Top 10 lithium cobalt oxide cathode material companies.