Principle and performance analysis of zinc carbon battery

Zinc carbon batteries are still useful as rechargeable aa batteries in devices with low losses or intermittent use, such as flashlights, remote controls, flash lights, toys, clocks, or transistor radios. The zinc carbon battery was the first commercial dry battery, developed from the technology of the wet Leclanché battery. Today, zinc carbon batteries have mostly been replaced by more efficient and safer alkaline batteries.
The working principle of zinc carbon battery
Zinc carbon battery is an Electrochemical system that uses zinc as the anode (the anode sends electrons to the circuit, so it is the anode, and the MangneseDioxide powder mixture and other materials are used as the cathode;
A nomonium chloride’s ElectrolyteJelly and Zinc chloride are dissolved in water as electrolyte; The carbon rod knife transmits the electrons from the external circuit back to the cathode. The cathode mixture in the battery also acts as a De-polarizer.
In simple terms, the container of zinc carbon battery is a zinc tank. Inside there is a layer of pasty liquid composed of NH4Cl and ZnCl2, which is separated from powdered carbon and manganese dioxide by a layer of paper.
These powders are packed around a carbon rod. In this dry battery, the outermost layer of zinc is the anode of the battery. In the half reaction, zinc is oxidized as shown in the following formula:
Zn(s) → Zn2+(aq) + 2 e
A graphite rod surrounded by powdered carbon and manganese dioxide serves as the cathde. Among them, manganese dioxide and carbon powder are mixed to increase the conductivity of the cathode. The cathode reaction is shown in the figure below:
2MnO2(s) + 2 H+(aq) + 2 e → Mn2O3(s) + H2O(l)
H+ comes from NH4+ (aq):
NH4+(aq) → H+(aq) + NH3(aq)
The generated NH3 can combine with Zn2+. Throughout the half-reaction, the oxidation state of manganese was reduced from +4 to +3. Although many side reactions may also occur, the overall reaction of zinc carbon battery can be expressed by the following formula:
Zn(s) + 2 MnO2(s) + 2 NH4+(aq) → Mn2O3(s) + Zn(NH3)22+(aq)
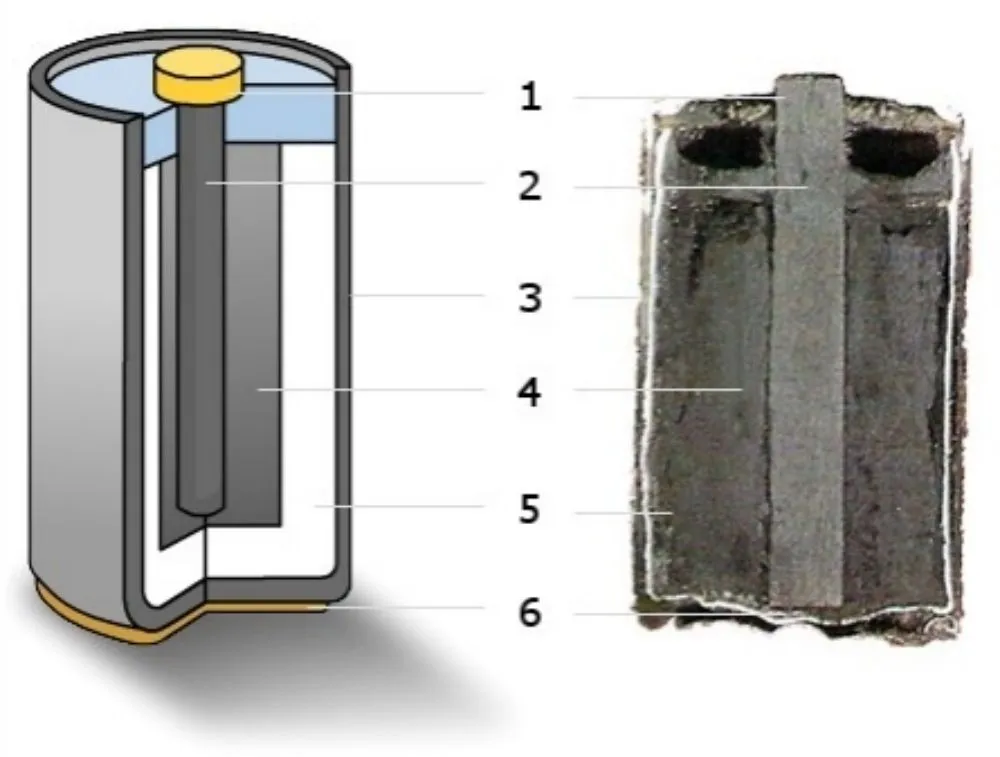
Cross section of zinc carbon battery. 1: metal cap (+), 2: graphite rod (cathde), 3: zinc shell (anode), 4: manganese dioxide, 5: moist ammonium chloride paste liquid (electrolyte), 6: metal end (- )
Rated voltage of zinc carbon battery
The standard voltage rating of a zinc carbon battery depends on the type of anode and cathode materials used in the battery. In zinc carbon battery, zinc is the cathode material, and manganese dioxide is the cathode material.
Zinc has an electrode potential of –0.7 volts and manganese dioxide has an electrode potential of 1.28. Therefore, the theoretical voltage of each battery should be -(- 0.76) + 1.23 = 1.99 V, but considering many practical situations, the actual voltage output of a standard zinc carbon battery does not exceed 1.5 V.
The uncertainty in the electromotive force is due to the complexity of the cathodic reaction, in contrast to the anode reaction (zinc terminal) which has a known potential. The consumption of side reactions and active reactants directly leads to an increase in the internal resistance of the battery and a decrease in the electromotive force of the battery.
Analyze the energy density of zinc carbon battery
The molar mass of the cathode material manganese dioxide is 87 g/mol. In the battery’s reaction, two electrons were found to reduce two manganese dioxide molecules. Thus, according to Faraday’s constant 28.6 Ah can be provided by a complete reduction of one mole or 87 grams of manganese dioxide. Therefore, 87/26.8 = 3.24 g of manganese dioxide are required to supply 1 Ah of electricity.
The molar mass of the anode material zinc is 65 g/mol. In the battery’s reaction, two electrons were found to oxidize a zinc atom. Therefore, according to Faraday’s constant, 28.6 Ah can be provided by fully oxidizing one mole or 65/2 grams or 32.5 grams of zinc. Therefore, 32.5/26.8 = 1.21 g of zinc are required to supply 1 Ah of electricity.
The total energy density of zinc carbon battery is 3.24 g/Ah + 1.21 g/Ah = 4.45 g/Ah =1 / 4.45 Ah/g = 0.224 Ah/g or 224 Ah/Kg. This is definitely a theoretical calculation, but in fact many other materials contained in the battery, such as electrolyte, carbon black, water, its weight cannot be omitted.
In addition to these, there are many other practical conditions in the battery that need to be considered. All things considered, a practical low discharge Leclanche battery has an energy density of 75 Ah/Kg, while for a heavy duty and intermittent discharge battery the energy density is around 35 Ah/Kg.
Advantages and disadvantages of zinc carbon battery
Advantages of zinc carbon battery
● The cost is quite low
● Available in a variety of shapes, sizes and capacities
● Long-term legacy reliability
Disadvantages of zinc carbon battery
● Very low energy density
● Poor temperature characteristics, poor low temperature characteristics
● Poor leakage resistance
● Does not perform effectively in high current consumption applications
● Poor voltage stability, voltage drops steadily with discharge
| Performance of zinc carbon battery | |
|---|---|
| Specific energy(wh/kg) | 40 wh/kg |
| Specific power(w/kg) | 150 w/kg |
| Cycle life (times) | >2000 times |
| Charging performance | 2~3C |
| Working temperature | -20℃-50℃ |
| Safety | No explosion and fire |
Application of zinc carbon battery
1. Zinc carbon battery for vehicles
In the past, lead-acid batteries were the most used batteries for electric vehicles. But lead-acid batteries are too heavy, take a long time to charge on one charge, and have a short driving distance, which cannot be compared with gasoline vehicles. These problems can be better solved by using zinc carbon battery instead. Compared with lead-acid batteries, zinc carbon battery has the characteristics of large capacity, high density and light weight, and has the basic conditions for electric vehicle power supply.
Now there are many lithium battery replacement lead acid used, the lithium battery is light in weight and small in size, and has excellent environmental protection performance. At present, as a high-end product, it is used as an electric motorcycle battery and an electric vehicle battery. The manufacturing cost is relatively high, and the cost is the advantage of zinc carbon battery.
2. Zinc carbon battery for power station peak regulation
Today’s society consumes more and more electricity, but the electricity consumption is not uniform. It is often used more during the day and less at night. There are peaks and valleys in electricity consumption, and the difference is getting bigger and bigger. The current solution to this problem is pumped hydro storage. Although this move can solve the problem, there are three disadvantages: first, it will take up a very large area, and hydropower stations cannot be built anywhere.
Second, it costs too much money. Building a hydropower station will cost hundreds of millions or billions. Third, there is a lot of waste. From electricity to water, and back from water to electricity, the power loss is about 40%. With zinc carbon battery, the problem will not be so serious. According to theoretical calculations, only one-tenth of the construction of a hydropower station is enough for land occupation and investment. As for the power loss, it does not exceed 20% from the small test data.