An introduction to battery cell types and the best battery cell

What is a cell in a battery
The anode is the negative electrode and it loses electrons during the oxidation process to the external circuit. Our commonly used batteries are 3.2v and 3.7v lithium ion battery cells.
The cathode is the positive electrode and it accepts electrons from the external circuit through reduction. A medium of transfer of charges between the anode and cathode is the electrode which consists of an alkaline, salt or acid solution.
A well-known example of the cell is the voltaic cell. The voltaic cell is made up of a carbon piece and a zinc piece plunged in a container containing water (h2o) and sulfuric acid (h2so4).
Why is a battery called a cell
A typical cell refers to one anode, one cathode and an electrolyte used to produce current and voltage. A collection of several cells is called a battery. Batteries can store electrical energy and also power electrical device. Batteries are composed of several cells that are connected in series to increase voltage and in parallel to increase current.
A cell can only deliver small quantities of voltage and current but the higher the number of cells combined together, the higher the amount of energy produced by the batteries.
Battery cell types and the best battery cell
Battery cell is classified into two categories which are called primary and secondary cell.Primary cells are cheap, portable, small and flexible to use. They are non-rechargeable batteries and must be discarded after single use because their electrodes are consumed as the battery discharges.
Some compact electronic devices such as watches, toys, mp3 players and radios use primary batteries. The most commonly used primary battery is the alkaline cell.

Another benefit of secondary cells is that they reduce waste production because they can be recharged and reused many times. They are also expensive compared to primary cells. Secondary cells have a longer life span than primary cells. They are used in several portable devices such as mobile phones and are also used as laptop lithium battery and car batteries. Examples include lithium ion, lead acid batteries, nickel-cadmium and nickel-metal hydride battery cell.
The best battery cell is the lithium-ion battery cell because they are reliable, have long life and have higher performance. Lithium-ion battery cell is also lighter in weight compared to battery cell of the same size.
The difference between a battery and a cell
The cell is a single enclosed electrochemical component that contains electrodes with a voltage difference. A battery cell consists of two or several cells connected together and fitted with devices such as a metallic case required to function properly. The difference between a battery and a cell is that a battery is simply the combination of two or more cells.
How many cells are in a 12 volt battery
A 12v battery has four single cells connected in series. The lead acid car battery consists of six cells in the casing. Each cell produces 3.2volts therefore along with the four battery cells, a total of 12.8volts.
What is lithium-ion cell used for
Lithium-ion battery cells have high energy density, are lightweight and come in a variety of sizes. They are reliable, have longer life and higher performance. They can be recharged as often as required when they run out. Currently, lithium-ion battery cells are the best and most commonly use.
They have several applications in the military, aviation, for several consumer electronics and used as electric vehicles and. They are also used to power electric motorcycles and used as wheelchair batteries. The common applications of lithium-ion battery cells are shown below:
Electric and recreational vehicle power
1.Lithium-ion batteries are lightweight and have high energy density. They are reliable and versatile therefore they can provide power for long journeys in electric/recreational vehicles.
Emergency power backup
Lithium-ion battery cell provide instant power that allows you safely shut down equipment in use. It is important for medical technology, communication and computers. It protects users from unreliable power supply.
Solar power storage
Seasonal variations affect the use of solar energy therefore; solar energy systems need to store energy to make them more reliable. Lithium-ion battery cells are fast charging, have low resistance and allow users maximize solar energy.
Surveillance systems for remote locations
Lithium-ion battery cells have large capacity and low self-discharge rates. They are suitable for applications requiring long-term low power consumption such as alarm systems.
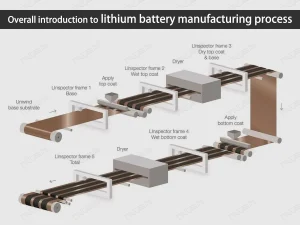
How lithium-ion battery cells are made
The lithium-ion battery cell consists of five components which are the anode, cathode, electrolyte, the shell and a separator. Lithium is a highly unstable element therefore it is combined with oxygen to form lithium oxide when used as the cathode inside the battery’s apparatus. A combination of lithium and oxygen gives lithium oxide which is more stable. Carbon is used as the anode and ether is commonly used as the electrolyte.
During the charging process, ions are removed from the cathode, travel through electrolytes and are transferred into the anode. During this process, energy is transferred into the anode. When the ions move through the electrolyte solution, the electrons move through the device connected to the battery and charge or power it. The voltage of the battery cell is determined by the electrochemical properties.Here’s a video about how to make a 12V 12Ah lithium battery.

Main types of lithium-ion battery cells
1.Lithium iron phosphate battery cell
2.Lithium titanate battery cell
3.Lithium manganese oxide battery cell
4.Lithium cobalt oxide battery cell
5.Lithium titanate battery cell
6.Lithium nickel manganese cobalt oxide battey cell
7.Lithium nickel cobalt aluminum oxide battery cell