How does a ultracapacitor discharge?
Ultracapacitor, also known as electrochemical capacitor, electric double-layer capacitor, gold capacitor, and Farad capacitor, is an electrochemical component developed in the 1970s and 1980s that uses polarized battery electrolyte to store energy.
Table of Contents

An introduction to ultracapacitor
Different from traditional chemical power sources, ultracapacitor are a kind of power source with special properties between traditional capacitors and batteries. They mainly rely on electric double layers and redox pseudocapacitors to store electrical energy. However, no chemical reaction occurs in the process of energy storage in ultracapacitor. This energy storage process is reversible, and it is precisely because of this that ultracapacitor can be repeatedly charged and discharged hundreds of thousands of times. The specific details of the ultracapacitor structure depend on the application and use of the ultracapacitor. These materials may vary slightly due to manufacturer or specific application needs. Common to ultracapacitor is that they contain a cathode electrode, a anode electrode, and a separator between the two electrodes, and the electrolyte fills the two pores separated by the two electrodes and the separator.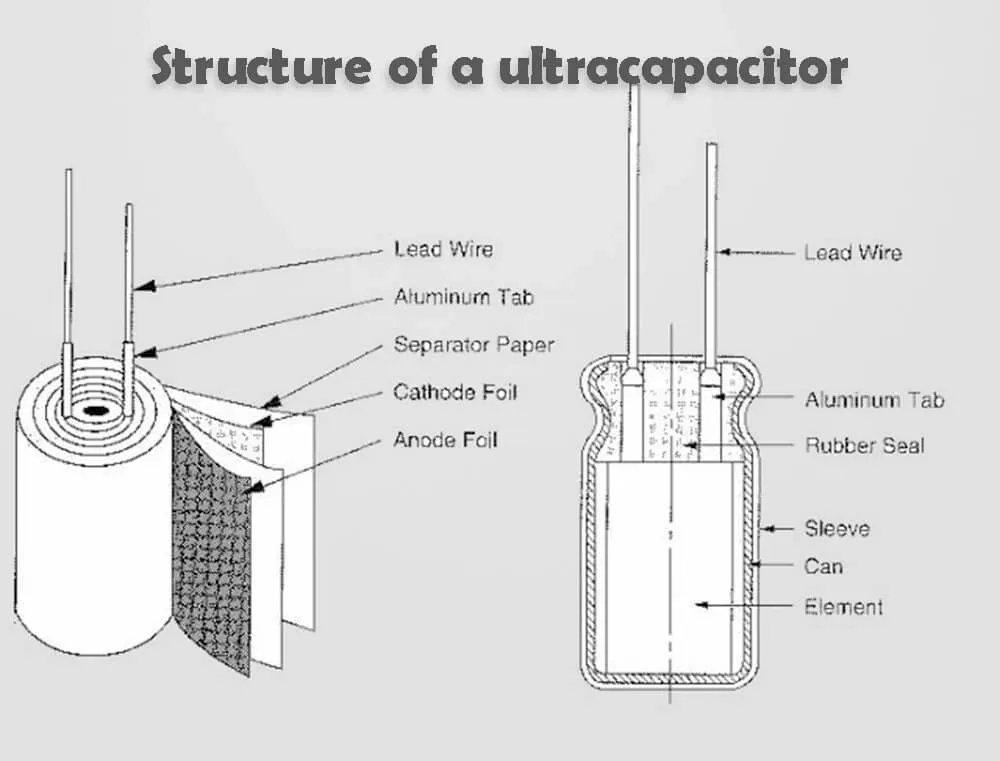 Ultracapacitor is composed of porous electrode materials with high specific surface area, porous batteries separators and electrolytes. Our website has another article top 5 battery separator companies on batteries separator that helps you learn more about batteries separator. The separator should meet the conditions of having as high ionic conductance as possible and as low as possible electronic conductance, and is generally an electronic insulating material with a fiber structure, such as a polypropylene film. The type of electrolyte is selected according to the properties of the electrode material.
Ultracapacitor is composed of porous electrode materials with high specific surface area, porous batteries separators and electrolytes. Our website has another article top 5 battery separator companies on batteries separator that helps you learn more about batteries separator. The separator should meet the conditions of having as high ionic conductance as possible and as low as possible electronic conductance, and is generally an electronic insulating material with a fiber structure, such as a polypropylene film. The type of electrolyte is selected according to the properties of the electrode material.
Classification of ultracapacitor
According to the different energy storage mechanisms of ultracapacitor, they can be divided into the following two categories: ● Electric double layer capacitance: As one ultracapacitor, it is generated by the confrontation of charges caused by the directional arrangement of electrons or ions at the electrode/solution interface. For an electrode/solution system, an electric double layer is formed at the interface of the electronically conducting electrode and the ionically conducting electrolyte solution. When an electric field is applied to the two electrodes, the anions and cations in the solution migrate to the positive and negative electrodes, respectively, forming an electric double layer on the surface of the electrodes. After the electric field is removed, the positive and negative charges on the electrodes attract the oppositely charged ions in the solution to stabilize the electric double layer, resulting in a relatively stable potential difference between the positive and negative electrodes. At this time, for a certain electrode, an anisotropic ion charge equivalent to the charge on the electrode will be generated within a certain distance (dispersion layer), so that it maintains electrical neutrality. When the two poles are connected to the external circuit, the charges on the electrodes migrate to generate a current in the external circuit, and the ions in the solution migrate into the solution to be electrically neutral. This is the charge and discharge principle of the electric double layer capacitor. ● Faraday quasi-capacitance: As an ultracapacitor, its theoretical model was first proposed by Conway. It is underpotential deposition of electroactive substances on the electrode surface and near surface or two-dimensional or quasi-two-dimensional space in bulk phase, and highly reversible chemical desorption and REDOX reactions occur to generate capacitance related to electrode charging potential. For Faraday quasi-capacitors, the process of storing charges includes not only the storage on the electric double layer, but also the redox reactions between electrolyte ions and electrode active materials. When the ions in the electrolyte (such as H+, OH-, K+ or Li+) diffuse from the solution to the electrode or solution interface under the action of external electric field, they will enter the bulk phase of the active oxide on the electrode surface through the REDOX reaction on the interface, so that a large amount of charge is stored in the electrode.
When discharging, these ions entering the oxide will return to the electrolyte through the reverse reaction of the above REDOX reaction, and at the same time, the stored charge will be released through the external circuit, which is the charging and discharging mechanism of Faraday quasi-capacitor.
When the ions in the electrolyte (such as H+, OH-, K+ or Li+) diffuse from the solution to the electrode or solution interface under the action of external electric field, they will enter the bulk phase of the active oxide on the electrode surface through the REDOX reaction on the interface, so that a large amount of charge is stored in the electrode.
When discharging, these ions entering the oxide will return to the electrolyte through the reverse reaction of the above REDOX reaction, and at the same time, the stored charge will be released through the external circuit, which is the charging and discharging mechanism of Faraday quasi-capacitor.
Advantages and disadvantages of ultracapacitor
Advantages of ultracapacitor
● The capacitance of the Farad level can be reached in a small volume; ● There is no need for special charging circuit and control discharge circuit; ● Compared with the batteries, overcharging and overdischarging will not have a negative impact on its life; ● Considering from the perspective of environmental protection, it is a green energy; ● The ultracapacitor can be welded, so there is no problem such as the weak contact of the batteries.Disadvantages of ultracapacitor
● Improper use may cause electrolyte leakage and other phenomena; ● Compared with aluminum electrolytic capacitors, its internal resistance is larger, so it cannot be used in AC circuits.The reason why ultracapacitor is called “ultra”
A ultracapacitor can be viewed as two inactive porous electrode plates suspended in an electrolyte. When power is applied to the plate, the cathode plate attracts the anode ions in the electrolyte, and the anode plate attracts the cathode ions, which actually forms two capacitive storage layers. The separated cathode ions are near the negative plate, and the anode ions are near the cathode plate. can be viewed as two inactive porous electrode plates suspended in an electrolyte. When power is applied to the plate, the cathode plate attracts the anode ions in the electrolyte, and the anode plate attracts the cathode ions, which actually forms two capacitive storage layers. The separated cathode ions are near the anode plate, and the anode ions are near the cathode plate. Ultracapacitor stores energy in the separated charges, and the larger the area used to store the charges and the denser the separated charges, the greater the capacitance. Compared with ultracapacitor, the area of traditional capacitors is the flat area of the conductor. In order to obtain a larger capacity, the conductor material is rolled for a long time, and sometimes a special structure is used to increase its surface area. In traditional capacitors, insulating materials are used to separate its two polar plates, generally plastic films, paper, etc. These materials are usually required to be as thin as possible. The area of the ultracapacitor is based on a porous carbon material whose porous structure allows it to reach an area of 2000 m2/g, with some measures enabling a larger surface area. The distance the ultracapacitor charges are separated is determined by the size of the electrolyte ions attracted to the charged electrodes. This distance (<10 Å) is smaller than what can be achieved with conventional capacitor film materials.
The huge surface area combined with the very small charge separation distance makes the ultracapacitor have a surprisingly large electrostatic capacity compared with the traditional capacitor, which is also its “ultra”.
The area of the ultracapacitor is based on a porous carbon material whose porous structure allows it to reach an area of 2000 m2/g, with some measures enabling a larger surface area. The distance the ultracapacitor charges are separated is determined by the size of the electrolyte ions attracted to the charged electrodes. This distance (<10 Å) is smaller than what can be achieved with conventional capacitor film materials.
The huge surface area combined with the very small charge separation distance makes the ultracapacitor have a surprisingly large electrostatic capacity compared with the traditional capacitor, which is also its “ultra”.
Controlling the discharge of ultracapacitor
The resistance of ultracapacitor prevents them from discharging quickly. The time constant τ of ultracapacitor ranges from 1 to 2s. It takes about 5τ to discharge fully for resistive and capacitive circuits, which means that it takes about 5 to 10s for a short circuit to discharge (due to the special structure of electrodes, they actually take several hours to discharge the residual charge completely). Ultracapacitor can be charged and discharged rapidly, peak current is limited only by their internal resistance, and even a short circuit is not fatal. In fact, it depends on the size of the capacitor cell. For matching loads, the small cell can put 10A, and the large cell can put 1000A. Another discharge rate limiting condition is heat. Repeatedly discharging at an aggressive rate will increase the capacitor temperature and eventually lead to an open circuit.Related post























