Lithium salt electrolyte types - advantages and disadvantages

At the same time, the electrolyte can also form a protective layer on the surface of the electrode material, which largely determines the capacity, operating temperature, cycle performance, power density, energy density and safety of lithium-ion batteries.
At present, lithium salt electrolytes used in lithium batteries mainly include inorganic lithium salt electrolyte and organic lithium salt electrolyte. This article mainly summarizes the common inorganic lithium salts and organic lithium salts, and reports the advantages and disadvantages of lithium salts electrolyte.
Inorganic lithium salt electrolyte
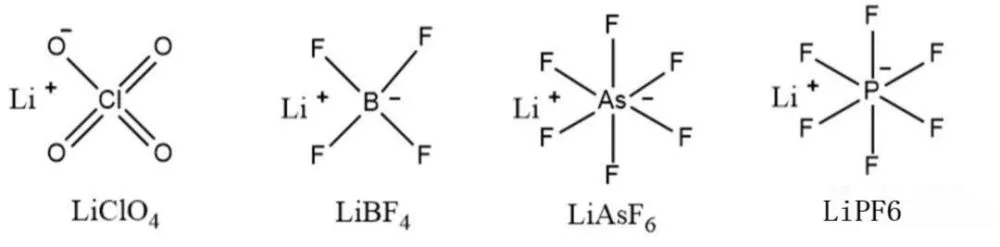
Generally speaking, inorganic lithium salts used in lithium-ion batteries generally have the advantages of low price, difficult decomposition, high potential tolerance, and simple synthesis. Common electrolyte inorganic lithium salts mainly include lithium perchlorate (LiClO4), lithium tetrafluoroborate (LiBF4), lithium hexafluoroarsenate (LiAsF6) and lithium hexafluorophosphate (LiPF6).
1.LiClO4
Lithium perchlorate (LiClO4) is a lithium salt electrolyte with relatively high solubility, so it exhibits relatively high ionic conductivity, and its room temperature ionic conductivity in carbonate organic solvents can reach 9 mS/cm. In addition, the electrochemical stability window of LiClO4 as lithium salt electrolyte electrolyte can reach 5.1 V vs. Li+/Li, which has relatively good oxidation stability, this property also enables the electrolyte to match some high-voltage cathode materials, thereby exerting the high energy density of lithium batteries.
In addition, LiClO4 has the advantages of simple preparation, low cost, and good stability, and has been widely used in laboratory basic research. However, since the Cl in LiClO4 is in the highest valence state +7, it is very easy to undergo oxidation-reduction reactions with the organic solvent in the electrolyte, which will cause safety problems such as combustion and explosion of lithium batteries. Therefore, LiClO4 is rarely used in commercial lithium batteries.
The molecular structure formula of inorganic lithium salt
2、LiBF4
Lithium tetrafluoroborate (LiBF4) has a relatively small anion radius (0.227 nm), therefore, the lithium salt electrolyte has a relatively weak coordination ability with lithium ions, it is easy to dissociate in organic solvents, which helps to improve the conductivity of lithium batteries, thereby improving battery performance.
However, it is precisely because its anion has a relatively small radius that it is easy to coordinate with the organic solvent in the electrolyte, which also leads to relatively low lithium ion conductivity, so LiBF4 is rarely used in normal temperature lithium batteries.
However, LiBF4 has relatively high thermal stability and is not easy to decompose at high temperature, so it is often used in high-temperature lithium batteries. Meanwhile, LiBF4 also exhibits good battery performance at low temperature, which is mainly due to the smaller interfacial impedance of the LiBF4-based electrolyte at low temperature.
In addition, LiBF4 has a certain corrosion resistance to the current collector Al. Therefore, LiBF4 is often used as an electrolyte additive for lithium-ion batteries, thereby increasing the corrosion potential of the electrolyte to the current collector Al.
3、LiAsF6
Lithium hexafluoroarsenate (LiAsF6) has the same ionic conductivity as LiBF4, and at the same time, the lithium salt electrolyte is not corrosive to the current collector Al. In addition, the electrochemical window of LiAsF6 lithium salt electrolyte can reach 6.3 V vs. Li+/Li, which is much higher than the electrochemical stability of general lithium salts. However, due to the highly toxic As element contained in LiAsF6, it is not often used in commercial lithium batteries.
4、LiPF6
Lithium hexafluorophosphate (LiPF6) is currently the most commonly used lithium salt electrolyte in commercial lithium batteries, which has relatively good ionic conductivity and electrochemical stability in aprotic organic solvents. In addition, the LiPF6 electrolyte can form a protective film with the current collector Al, thereby reducing the corrosion of the electrolyte to the current collector Al.
More importantly, the carbonate electrolyte based on LiPF6 lithium salt electrolyte can form a layer of solid electrolyte interface (SEI) on the graphite anode, in this way, the adverse reaction between the electrolyte and the graphite anode is protected, and the lithium-ion battery has good long-term cycle performance.
However, LiPF6 lithium salt electrolyte has poor thermal stability. In addition, it easily reacts with trace amounts of water to produce strong acid PF5. PF5 easily undergoes side reactions with organic solvents in the electrolyte, resulting in attenuation of battery performance. Major LiPF6 companies in China include top 10 lithium hexafluorophosphate companies.
Organic lithium salt electrolyte
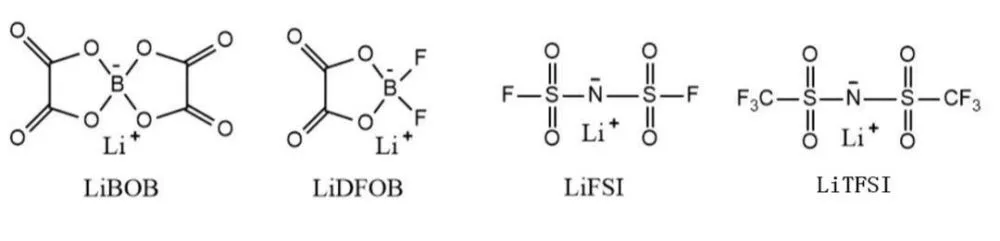
Compared with inorganic lithium salts, organic lithium salts commonly used in lithium-ion batteries can be considered to be regulated by adding electron-withdrawing groups to the anions of inorganic lithium salts. Common electrolyte organic lithium salts mainly include lithium bisoxalate borate (LiBOB), lithium difluorooxalate borate (LiDFOB), lithium bisdifluorosulfonyl imide (LiFSI) and lithium bistrifluoromethylsulfonyl imide (LiTFSI) .
The molecular structure formula of organic lithium salt
1、LiBOB and LiDFOB
Lithium bisoxalate borate (LiBOB) was synthesized by Kang Xu et al. 4 and used as lithium salt electrolyte in lithium batteries, LiBOB lithium salt electrolyte has the advantages of high ionic conductivity, wide electrochemical stability window, good thermal stability, and good cycle stability.
In addition, studies have shown that it can form a stable passivation film with the current collector Al to protect Al from electrolyte corrosion. However, LiBOB has the obvious disadvantage of its low solubility in aprotic solvents, leading to low conductivity of electrolytes composed of it, which limits the rate performance of batteries based on this salt.
In order to overcome the disadvantages of poor solubility and low ionic conductivity of LiBOB, Zhang et al. 5 took a part of LiBOB and LiBF4 lithium salt electrolyte to synthesize another new type of lithium salt electrolyte, lithium difluorooxalate borate (LiDFOB). Studies have shown that LiDFOB has a much higher ionic conductivity than LiBOB;
In addition, it has good electrochemical stability and good compatibility with cathode and anode. In addition, batteries based on the lithium salt electrolyte also exhibit good low-temperature performance. Based on the above advantages, LiDFOB is widely used in current lithium batteries.
2、LiFSI and LiTFSI
Lithium bisfluorosulfonyl imide (LiFSI) has the advantages of high ionic conductivity and low sensitivity to water. In addition, LiFSI has a higher decomposition temperature than LiPF6, and has relatively better safety. However, LiFSI is highly corrosive to the current collector Al, thus limiting its application in Li-ion batteries to some extent. Here are top 5 LiFSI companies in China.
Lithium bis(trifluoromethylsulfonyl)imide (LiTFSI) is another organic lithium salt developed by Michel Armand, its anode ions are composed of nitrogen (N) atoms with strong electronegativity and two sulfur (S) atoms connected with strong electron-attracting groups (CF3). This structure disperses negative charges, making it easier for cathode and anode ions to dissociate, thereby significantly improving its ionic conductivity.
Adding other lithium salts that do not corrode the current collector to LiTFSI, introducing long-chain perfluorinated groups, and adding additives to LiTFSI can significantly increase the corrosion potential of LiTFSI to the current collector. Although these two lithium salt electrolytes have the characteristics of corroding the current collector Al, however, due to the advantages of high ionic conductivity, good thermal stability, and good electrochemical stability, it has been widely used in lithium-ion batteries, all-solid-state polymer lithium batteries, and lithium-sulfur batteries.
Conclusion
As an important part of lithium batteries, lithium salt electrolyte not only provides and transmits lithium ions, but also determines the overall performance of lithium batteries to a certain extent.
A detailed understanding of the advantages and disadvantages of existing lithium salt electrolytes will provide a good reference and impetus for the development of new lithium salt electrolytes. If you are interested in Lithium related companies, please refer to the top 10 lithium salt enterprises in China