Advantages and disadvantages of potassium ion battery vs lithium
As demand for lithium resources increases and supply capacity declines, ultimately, human needs will not be met in the future.
Therefore, there is an urgent need to develop new energy storage devices, such as sodium-ion batteries (SIBs), potassium ion batteries (PIBs), etc., it is hoped that it can be used as a complement to LIBs in large-scale energy storage applications, thereby minimizing concerns about lithium resource shortages.
We also discussed the comparison of lithium vs sodium battery before.

As demand for lithium resources increases and supply capacity declines, ultimately, human needs will not be met in the future. Therefore, there is an urgent need to develop new energy storage devices, such as sodium-ion batteries (SIBs), potassium ion batteries (PIBs), etc., it is hoped that it can be used as a complement to LIBs in large-scale energy storage applications, thereby minimizing concerns about lithium resource shortages. We also discussed the comparison of lithium vs sodium battery before.
In this article, I will introduce the working principle, advantages and disadvantages of potassium ion battery and compare the similarities and differences of lithium-ion batteries to see if potassium ion battery can replace lithium-ion battery.
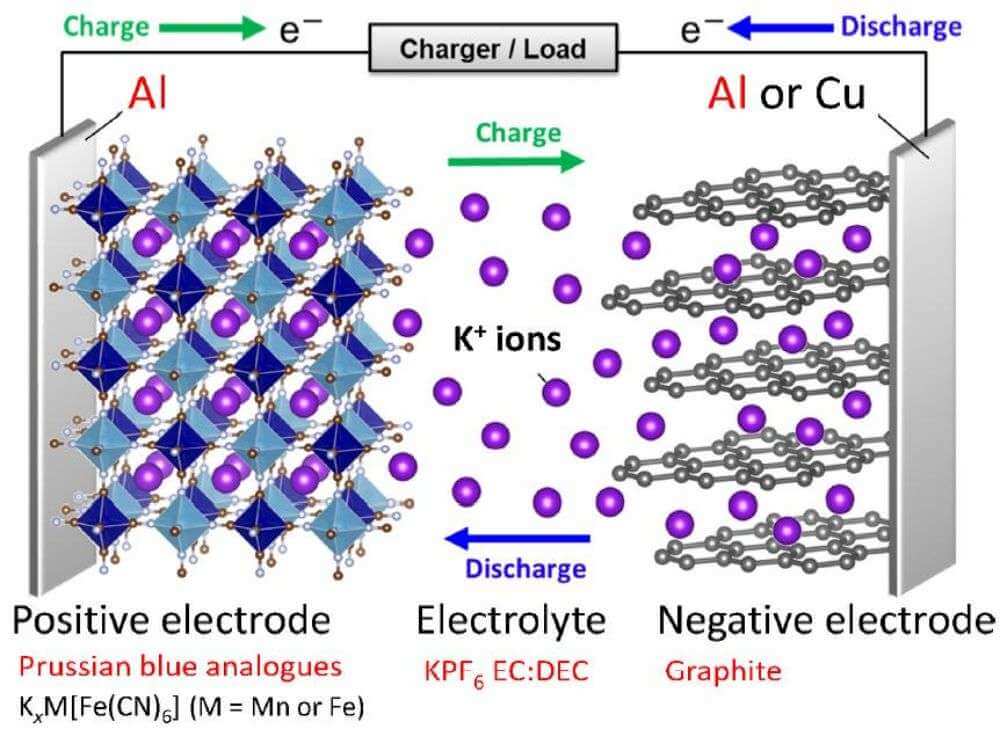
How a potassium ion battery works
From the periodic table of elements, we can know that lithium and potassium belong to the first main group, so the properties of these two elements are very similar. The potassium ion battery is a “rocking chair” battery with the same principle as the lithium ion battery. Because the insertion and extraction of cations in the positive and negative materials is very similar to a chair rocking back and forth, it is likened to a “rocking chair”.
Advantages and disadvantages of potassium ion battery
The advantage of potassium ion battery is that high-priced raw materials such as lithium, cobalt, and copper used in lithium-ion batteries can be replaced with inexpensive and abundant raw materials such as potassium, iron, and aluminum. Moreover, potassium has less risk of fire than lithium and can also improve safety.
The potassium ion battery is rich in raw materials, has the advantages of high energy density, fast ion transport in the electrolyte, and low cost, and has become the first choice for replacing lithium ion batteries. Moreover, compared with lithium, potassium has less risk of fire and improved safety performance.
Potassium ion batteries based on abundant potassium resources have demonstrated several advantages, including low cost and high operating voltage, while having significant potential for large-scale energy storage. However, their main disadvantages are low specific energy, cycle life, etc., which hinder their further applications.
In addition, because of the larger radius of potassium ion, the reactivity of metal potassium is higher, which leads to the great difference between the electrode material design and the manufacture of full battery of potassium ion battery and other alkali metal ion batteries. However, its application prospects in the future are still worthy of attention, especially in large-scale energy storage fields that are less sensitive to battery quality and volume but have higher cost requirements.
Comparison of potassium ion battery and lithium-ion battery
● Battery cost
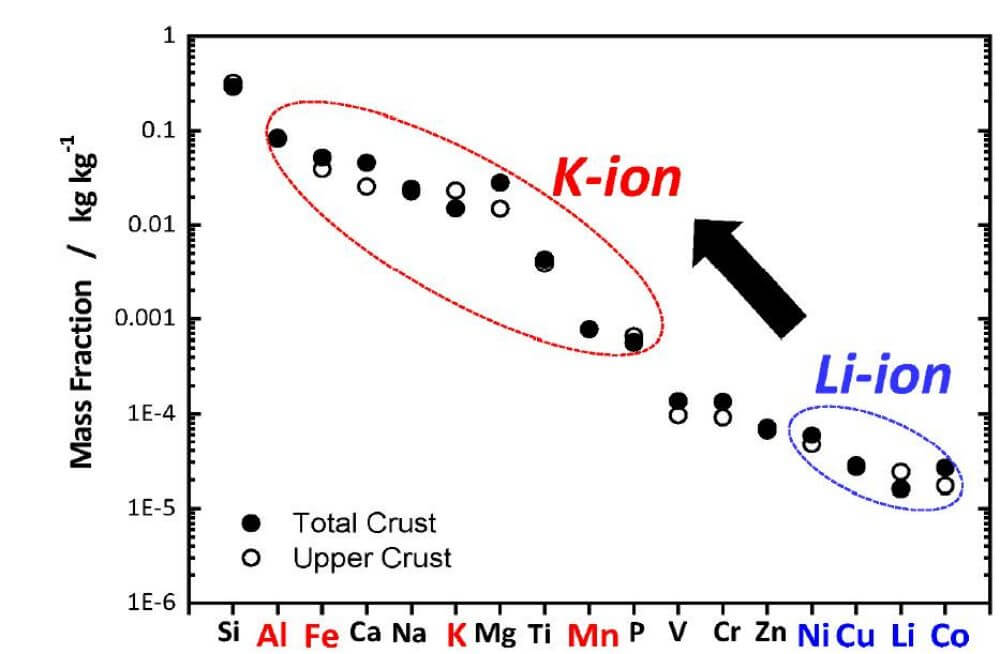
Potassium ions are more abundant than lithium ions, and potassium resources are very rich, which can be said to be “inexhaustible” and the price is very low. In addition, since potassium does not thermodynamically form Al-K intermetallics, this suggests that aluminum foil can replace copper foil as the negative current collector in such batteries, while aluminum is generally less expensive than copper, which can also reduce battery costs. If you want to know about the aluminum foil industry information, you can refer to battery aluminum foil manufacturers.
Element Abundance in the Earth’s Crust
● Battery energy density
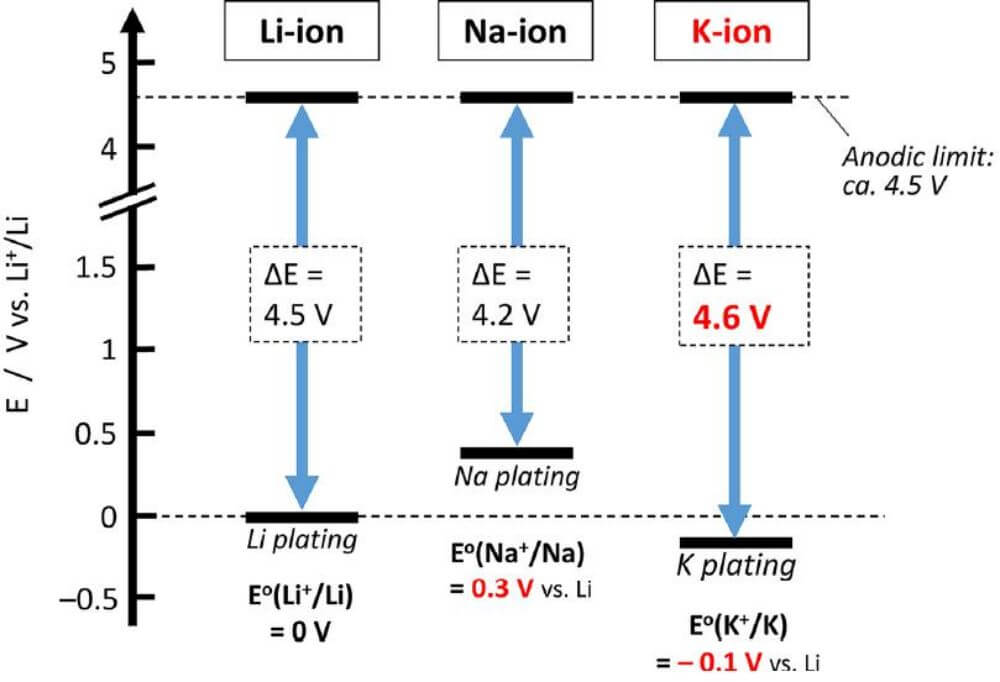
The lower standard electrode potential of potassium in terms of energy stored per unit volume of battery means that a lower cut-off potential of the negative electrode can be used, in addition to the absence of metallic potassium deposition.
The same as the first main group elements, the electrode potentials of lithium, sodium and potassium are -3.04V, -2.71V and -2.93V respectively, and the voltage of the battery is equal to the potential difference between the positive and negative electrodes.
Therefore, the potentials of lithium and potassium are more similar, and the potential ion battery has the potential to operate at higher voltages over a wider voltage range, and higher voltage operation means the possibility of higher battery energy density.
The available potential windows for lithium-, sodium-, and potassium-based batteries assuming the use of nonaqueous electrolyte solutions of carbonate solvents.
● Battery power density
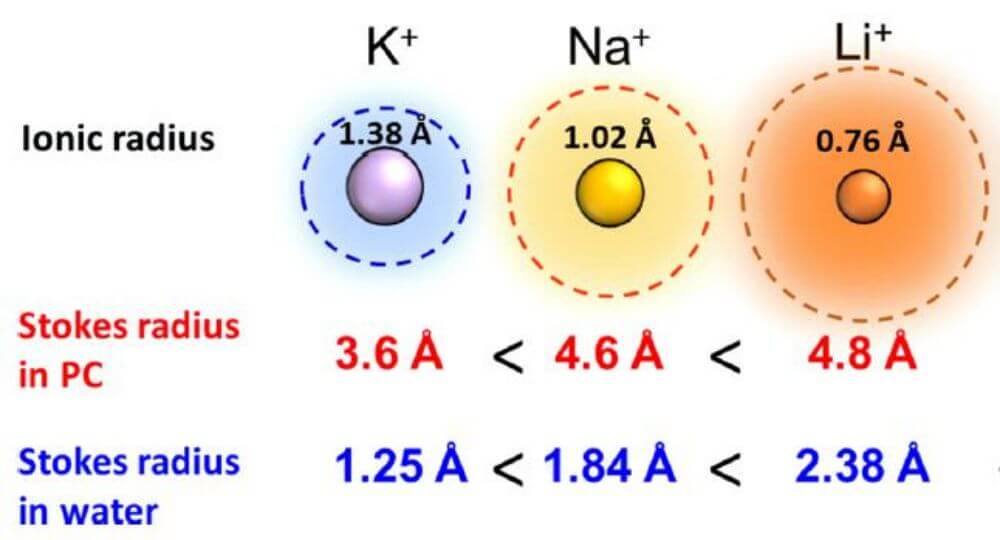
In terms of the rate at which energy output per unit weight of battery can be carried out, potassium ions have the smallest Stokes radius in the electrolyte solution, that is, potassium ions can diffuse rapidly in the electrolyte solution to provide high power density.
In a related study, the researchers found that potassium ions with a larger atomic radius can intercalate into graphite and provide a specific capacity of about 250mAh/g, in which potassium is very similar to lithium.
In addition, the problem of alkali metal negative electrodes and dendrites, for potassium ion battery researchers also found that an innovative idea can be used to solve: If a sodium-potassium alloy is used as the negative electrode, it is liquid at room temperature, and the liquid negative electrode has no problem of dendrite formation at all, and the metal deposited during the charging process will become liquid.
Moreover, the immiscibility of the sodium-potassium alloy and the organic electrolyte makes the liquid metal negative electrode not require a solid electrolyte, so that the battery with the alloy as the negative electrode can operate at room temperature.
Due to the “relative difference” in the sodium-potassium potential (the potassium potential is more negative, it is relatively easier to lose electrons), the active metal in the sodium-potassium alloy is the metal potassium, and the sodium metal does not participate in the reaction of the battery. However, sodium plays the role of liquefying the potassium metal, and the sodium-potassium alloy has a specific capacity of 579 mAh/g as a potassium anode.
Comparison of Shannon ionic radius and Stokes radius of Li+, Na+ and K+ ions in water and PC
Can potassium ion battery replace lithium-ion battery
Lithium-ion batteries are still the current mainstream energy storage devices, like lithium powerwall battery and lithium portable power station, the demand for lithium-ion batteries is increasing. The shortage and uneven distribution of lithium resources have severely limited the wide and sustainable application of such batteries.
In recent years, sodium-ion batteries and potassium ion batteries have attracted extensive attention due to their abundant metal resources. Although the price of potassium metal is relatively high compared to sodium, the price of K2CO3 is similar to that of Na2CO3 while being much cheaper than Li2CO3.
In addition, aluminum foil can be used as a current collector in potassium ion battery to replace copper foil in lithium ion battery, this can not only significantly reduce the price of the potassium ion battery, but also reduce the weight of the current collector and solve the problem of over-discharge.
Although the atomic radius of potassium (1.38 Å) is larger than that of lithium (0.68 Å) and sodium (0.97 Å), the Stokes radius of potassium ion (3.6 Å) is smaller than that of lithium ion (4.8 Å) and sodium ion (4.6 Å) , this means that potassium has the highest ionic mobility and ionic conductivity in the electrolyte.
Based on the above advantages, the potassium ion battery is expected to replace the lithium-ion battery as the next-generation high-performance energy storage device. However, the potassium ion battery still faces some challenges, which hinder its commercialization. First, the larger K+ makes the volume expansion of the potassium ion battery more severe than other alkali metal ion batteries during the charge/discharge process, which leads to the collapse of the crystal structure of the electrode material and the pulverization of the electrode.
Second, the low bulk diffusion rate of potassium ions in the electrode material limits its rate capability improvement. Finally, the electrolyte in the potassium ion battery suffers from severe decomposition and some side reactions due to the high potential of the K+/K redox. Therefore, it is crucial to further explore suitable electrode materials and develop potassium ion battery technology.

























1 thought on “Advantages and disadvantages of potassium ion battery vs lithium”
I used to bbe abpe to finnd good information from
your articles.