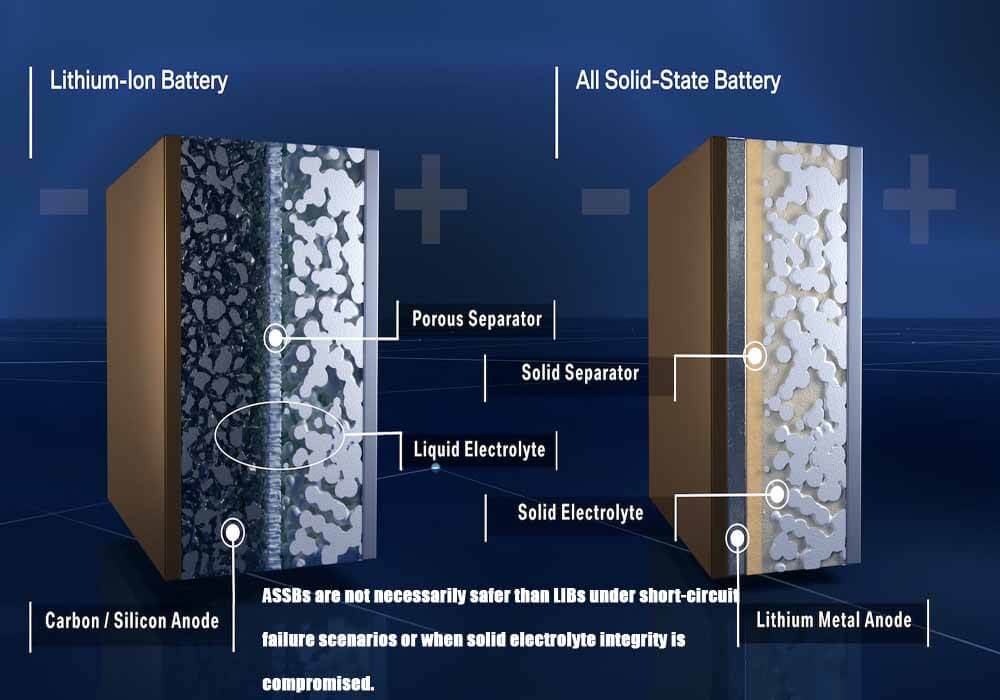
The effect of electrolyte on the safety of solid state batteries

The safety benefits of this solid state electrolyte alternative are widely recognized. However, the broader safety of high-energy-density Li-metal anode solid state battery has not been rigorously examined. This work broadens the discussion of solid-state battery safety using thermodynamic models.
The heat release and temperature rise upper bounds for several cell-level failure scenarios and battery structures are explored, including a direct comparison with lithium batteries. The thermodynamic effects of adding liquid electrolytes in solid-state batteries were also evaluated.
Three thermal runaway modes
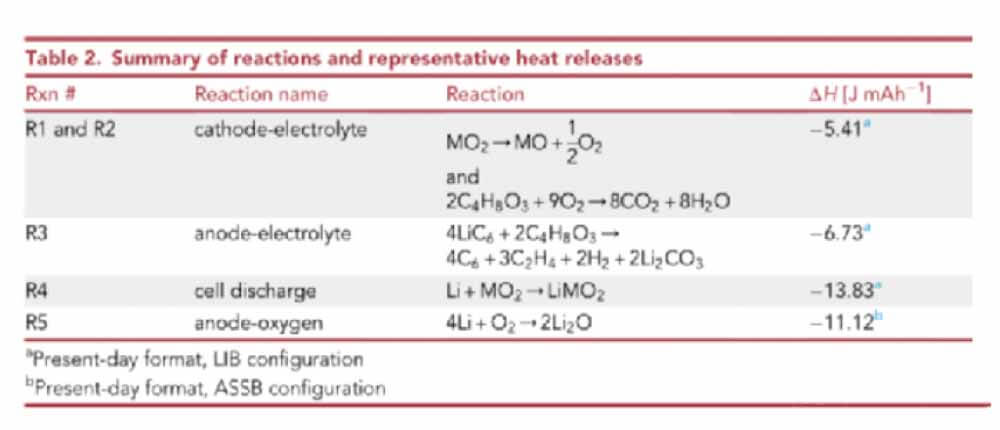
A: Thermal runaway caused by external heating leads to the decomposition of the positive electrode and the liquid electrolyte reaction with the O2 generated by the positive electrode. For liquid lithium ion batteries (LIB), the liquid electrolyte reaction of lithiated graphite occurs.
B: An internal short circuit that maintains the mechanical integrity of the battery, resulting in the conversion of stored electrochemical energy into heat.
C: Mechanical failure of the solid electrolyte, the gas generated at the positive electrode can freely react with the lithium at the negative electrode.
The results show that adding a sufficiently small amount of liquid electrolyte is a reasonable step to achieve the commercialization of solid-state batteries (SSBs) even with safety concerns in mind. The work also shows that the temperature rise of SSBs or all-solid-state batteries (ASSBs) may be higher than that of LIBs of equivalent areal capacity due to their high energy density.
Figure 1 :Structure of SSB and LIB
Out of control method A
Thermal runaway caused by an external heat source. Failure of the solid electrolyte separator is not considered. For ASSSB and SSB, the chemicals produced at the positive electrode are prevented from reaching the lithium at the negative electrode.
Alkyl carbonate electrolytes are known to react with the positive electrode, while in LIBs, the lithiated graphite negative electrode releases heat.
Assumption
ASSB: In the absence of liquid electrolyte to promote the reaction, the temperature of O2 released from the positive electrode is higher. The solid electrolyte, due to its high density, is an effective gas barrier to prevent the contact between the anode Li and the O2 released at the cathode. It is assumed that there will be no significant heat release in this scenario.
SSB: The liquid electrolyte exists in the pores of the positive electrode, which can catalyze the release of O2 from the positive electrode at high temperature (lower than ASSB).
O2 is consumed by reacting with the liquid electrolyte, resulting in the release of heat and the production of CO2 and H2O gases. The solid electrolyte prevents the gas from coming into contact with the negative electrode.
LIB: The liquid electrolyte exists in the pores of the positive electrode, separator and negative electrode. The O2 released at high temperature is consumed by the reaction with the liquid electrolyte.
The unreacted liquid electrolyte reacts with the lithiated negative electrode. The heat release due to the degradation of the initial solid electrolyte interphase (SEI) layer is neglected as it typically only accounts for 5% of the heat release from the reaction of the anode with the liquid electrolyte.
Thermal runaway mode B
Short-circuit failure occurs due to the penetration of the dendrite through the electrolyte, releasing all the stored electrochemical energy in the form of heat.
Assumption
Like runaway mode A, the solid-state electrolyte is an effective gas barrier. It is assumed that the entire stored (electro)chemical energy of the battery is converted into thermal energy.
In order to distinguish the discharge reaction from the heat release of other reactions considered in runaway mode A, the rate of other reactions (eg, cathode decomposition and subsequent reaction with liquid electrolyte) is zero.
Thermal runaway mode C
Mechanical failure of the solid electrolyte, all chemical species generated on the positive side are free to oxidize the lithium of the negative electrode.
This scenario is only applicable to ASSB to show the potential heat release of the structure, the O2 generated at the positive electrode can freely react with the lithium metal negative electrode.
Source and size of heat production
Analysis conclusion
Electrolyte integral and heat
Summary of reactions and representative heat releases
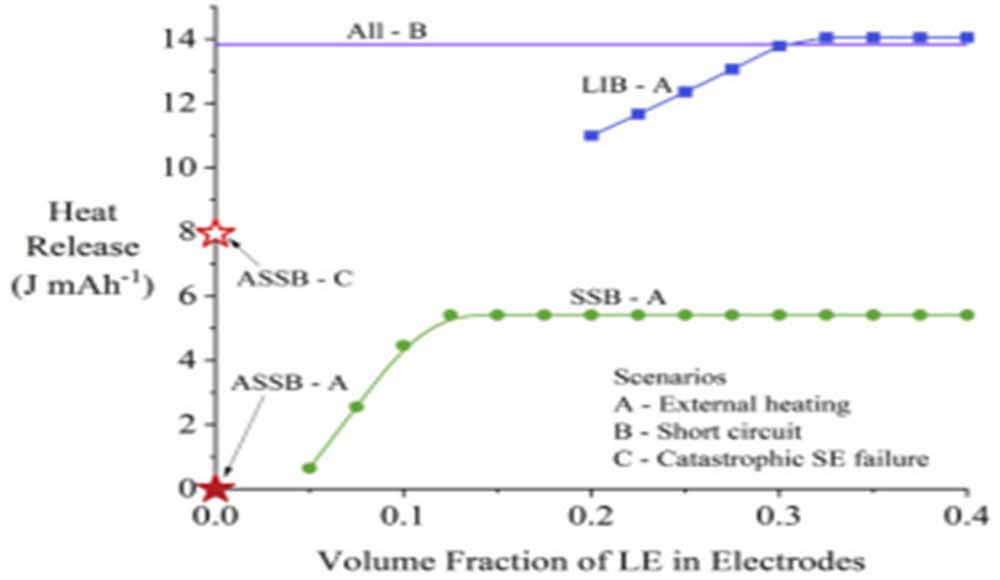
Figure 2: Relationship between heat release and volume fraction of liquid electrolyte
Figure 2 shows the potential thermodynamic heat release of LIB and SSB as a function of liquid electrolyte volume fraction. SSB with a small amount of liquid electrolyte added generates more heat than ASSB, but runaway mode A is still much less than LIB when subjected to external heating.
If the solid electrolyte can effectively insulate the positive and negative electrodes, the heat release from the ASSB is not expected even at high temperature (runaway mode A, solid star in Figure 2).
For SSB, a plateau is formed when the volume fraction edge is above 0.125, at which point all the O2 produced by the cathode has reacted with the liquid electrolyte; the excess liquid electrolyte may be discharged. There will be a plateau on the volume fraction (R0.3) of the LIB due to the additional reaction of the liquid electrolyte with the anode Li (the liquid electrolyte can be in contact with the anode Li in the LIB).
Even at a low liquid electrolyte volume fraction (0.2 in Fig. 2), the heat release from LIB is almost double the estimated value of SSB. At more typical values, namely 0.3 volume fraction of liquid electrolyte in LIB positive/negative electrodes and 0.1 volume fraction of liquid electrolyte in SSB positive electrode, the potential heat release of SSB is about one-third that of LIB.
It is also worth noting that since the oxygen loss from the NMC cathode is endothermic, the heat released is negligible when the volume fraction of the liquid electrolyte is less than 0.08. ASSB and SSB may not be any safer than LIB in other runaway ways.
The horizontal line near 14 JmAh-1 in Figure 2 shows the heat release for all structures under short-circuit failure (runaway mode B). The heat released by runaway mode B only depends on the battery capacity. Therefore, short-circuit faults generate the same heat release in ASSBs, SSBs, and LIBs.
Furthermore, if the solid electrolyte fails mechanically (runaway mode C), allowing O2 on the positive side to reach metallic lithium, the heat release for reactions 1 and 5 can be enormous, shown as a hollow star in Figure 2.
Energy density and heat release
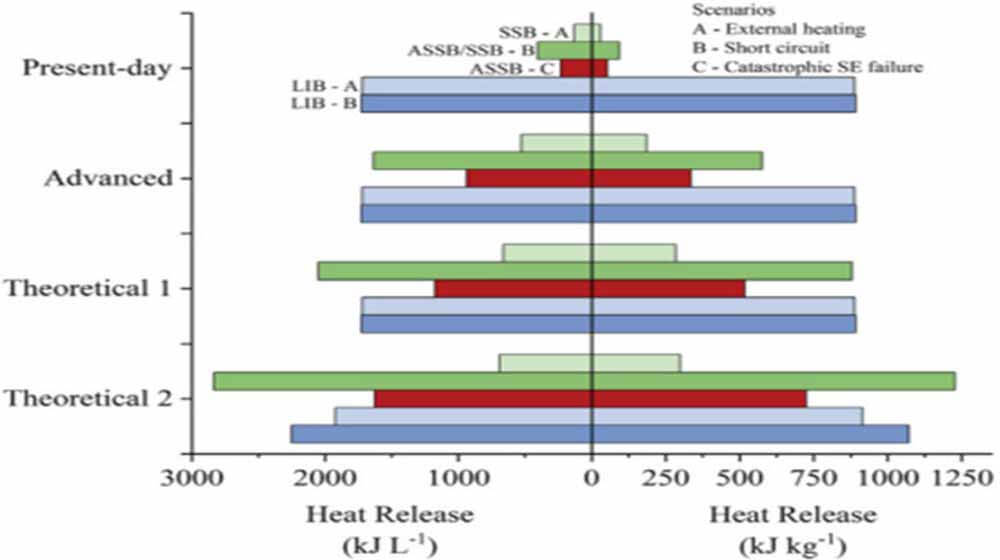
Figure 3: The relationship between the integral fraction of electrolyte in the electrode and the heat release
As batteries achieve higher energy density by reducing solid electrolyte thickness and increasing cathode loading, the same amount of heat is released on a smaller mass or volume.
Figure 3 compares the heat release by weight and volume. Current solid-state electrolytes tend to be unrealistically thick, resulting in low energy storage densities, both in terms of weight and volume.
As the structure and energy density approach advanced theoretical values, the potential heat release per unit mass and volume of the SSB in runaway mode B increases proportionally. The important point here is that the LIB heat release of runaway mode A tends to be similar to that of runaway mode B, whereas they are quite different for SSB designs with limited liquid electrolytes.
For SSB and LIB, the energy density drives the potential heat release of runaway mode B. Runaway mode C shows the potential for a significant thermal release of potential chemical energy in ASSBs under solid-state electrolyte mechanical failure conditions, although the likelihood of such an event is currently unclear.
Battery structure or potential temperature rise to increase energy density
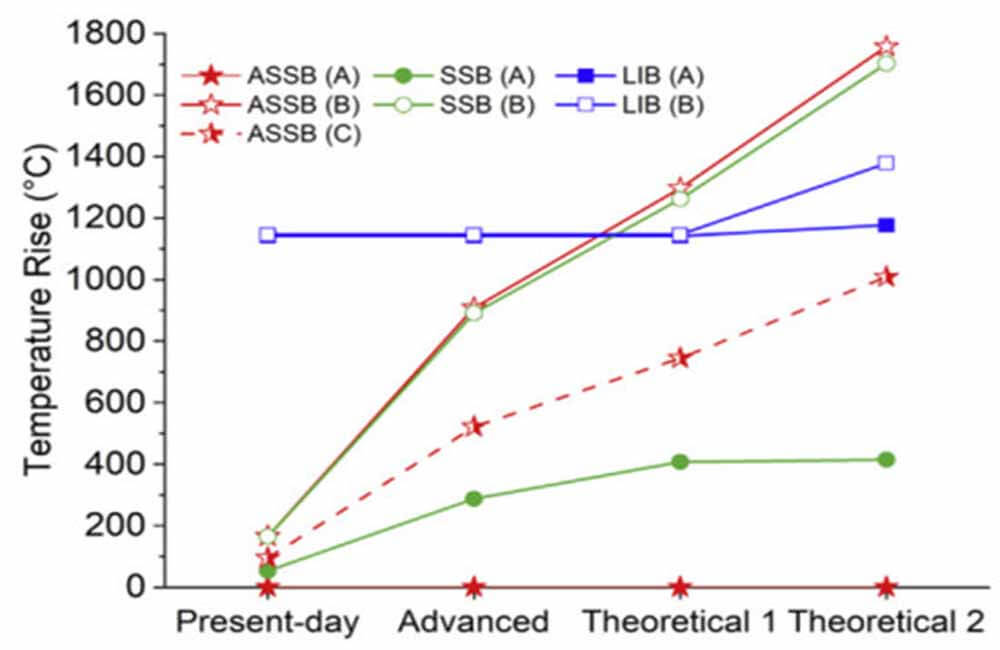
Figure 4: Potential temperature rise based on battery structure or increased energy density
Depending on the runaway pattern, the temperature rise of ASSB and SSB may be higher or lower than that of LIB. The ASSB does not experience a temperature rise due to an exothermic reaction when heated externally (runaway mode A).
Conversely, as the energy density increases from the present to the theoretical 2 format, the potential temperature rise of the SSB subjected to external heating increases from 53 °C to 415 °C due to the presence of liquid electrolyte reactions. This shows that increasing the energy density has a large effect on the temperature rise.
LIB experiences a higher potential temperature rise (~1,100 °C) than SSB and ASSB, with a slight increase in higher energy density structures. For LIBs, the potential temperature rises for runaway modes A and B are somewhat larger than typically observed because the importance of liquid electrolyte venting in reducing potential temperature rises is neglected.
Figure 4 shows an important point, as the energy density increases, the potential temperature rise of SSB and ASSB in short-circuit fault (runaway mode B) exceeds that of LIB, suggesting that the safety of SSB and ASSB may be low in this scenario in LIB.
Because Li has been shown to grow through LLZO and short-circuit the cell, short-circuiting is a relevant failure mechanism to consider. The increase in potential temperature rise is due to the smaller thermal mass in the SSB and ASSB structures and the relatively low heat capacity of LLZO.
Figure 4 shows that as solid-state electrolytes become thinner, preventing internal short circuits and diaphragm failures is critical to safety—more important than reducing liquid electrolytes. Even with the increased energy density, the temperature rise of the SSB (runaway mode A) is much lower than that of the LIB, and possibly lower than the temperature of cascade propagation (thermal runaway of adjacent cells). Furthermore, in ASSB (runaway mode C), the potential temperature rise of the Li+O2 reaction is close to that of LIB in runaway mode A when the solid electrolyte fails.
In conclusion, this analysis suggests that high-energy-density ASSBs and SSBs may not offer significant safety advantages relative to LIBs, and that ASSB/SSB development should focus on solid-state electrolyte integrity and protection against short circuits.
Outlook
This work uses thermodynamic modeling to challenge common assumptions about battery safety. The safety evaluation uses LLZO solid electrolyte to evaluate the safety performance of solid-state battery SSB with liquid electrolyte, all-solid-state battery ASSB without liquid electrolyte, and conventional liquid lithium battery LIB.
It is often claimed that ASSB is more secure than LIB. Studies have shown that this is true under external heating failure scenarios, but that ASSBs are not necessarily safer than LIBs under short-circuit failure scenarios or when solid electrolyte integrity is compromised. In future high-energy-density structures involving lithium metal anodes, ASSBs are expected to experience higher temperature rises than LIBs because the same amount of heat is generated on a smaller mass and volume.
Short circuits are a common problem for ASSBs because lithium dendrite can grow through the solid-state electrolyte and reach the cathode. As solid-state electrolytes become thinner under the requirement of increasing energy density, the ability to prevent dendrite growth generally decreases.
Preventing Li dendrite growth into the solid-state electrolyte and ensuring that reactive species do not cross the solid-state electrolyte are key safety issues that need to be overcome before ASSB is commercialized. The present work clearly shows that the evolution of energy density, solid-state electrolyte thickness, and battery design affects potential safety issues.
This work is the first quantitative analysis of the safety of SSBs containing liquid electrolytes. Adding liquid electrolytes to SSBs can improve interfacial resistance, but is sometimes thought to reduce the safety of SSBs to the point that it is not a commercially viable solution scenario.
This work quantifies that an SSB design with a small amount of liquid electrolyte in the cathode can improve safety characteristics compared to current LIB designs in scenarios where a typical thermal runaway reaction occurs.
The possibility of accidental thermal release due to the release of stored energy is common in all structures, and the consequences depend mainly on the energy density, which can vary widely among SSB structures. As the push for higher energy density batteries continues, the maximum temperature at failure will increase, which is expected to have a major impact on safety. If you are curious about the relevant solid-state battery manufacturers, you can browse Top 10 solid-state battery companies.