Home » lithium ion battery knowledge » Are lithium batteries safe – battery risks and comparison
Are lithium batteries safe - battery risks and comparison
With the continuous development of lithium battery technology, lithium batteries are widely used in many fields such as consumer electronics products, electric bicycles and other mobility products, electric vehicles (EV) and energy storage solutions.
Are lithium batteries safe has gradually become a concern of people, which will be introduced and compared in this article.

How do lithium batteries work?
Before getting to the problem of are lithium batteries safe, the following will introduce the working principle of lithium battery from three parts: charging process, discharging process and battery protection board:
1. Lithium battery charging process
The cathode of the battery is generated by lithium ions, and the generated lithium ions “jump” into the electrolyte from the cathode, “crawl” through the small tortuous holes in the separator through the electrolyte, and move to the anode. The electrons at the anode are bonded together.
● The reaction on the cathode is: LiCoO2==charging==Li1-xCoO2+Xli++Xe (electron)
● The reaction on the anode is: 6C+XLi++Xe=====LixC6
During the charging process, Li+ comes out of the cathode LiCoO2, enters the electrolyte, and moves to the anode under the action of the external electric field added by the battery charger. Enter the anode composed of graphite or coke C in turn, and form LiC compound at the anode.
2. Lithium battery discharge process
When discharging, both electrons and Li+ act at the same time, in the same direction but in different paths.
The electrons run from the anode to the cathode through the external circuit. Lithium ion Li+ “jumps” into the electrolyte from the anode, “climbs” through the curved small hole on the diaphragm, “swims” to the cathode, and combines with the electrons that have run over. What we usually call the battery capacity refers to the discharge capacity.
3. Battery protection board
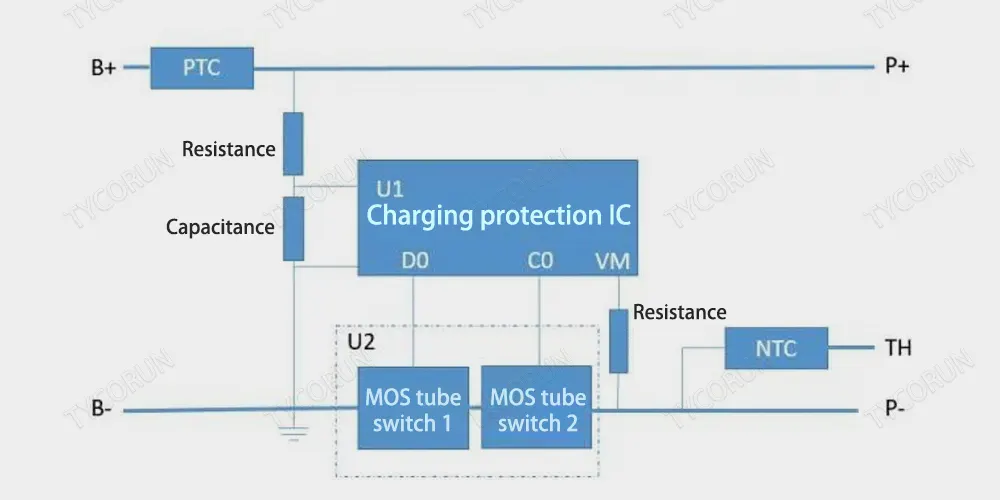
As the name implies, the battery protection board is mainly an integrated circuit board that protects rechargeable batteries (generally referred to as lithium batteries).
The reason why the lithium battery needs protection is that the material of the lithium battery itself determines that it cannot be charged and discharged by overcharge, overdischarge, overcurrent, short circuit and ultra-high temperature, so the lithium battery always has a protection board and a current fuse .
The picture above shows the battery board protection circuit.
PTC: Positive temperature coefficient thermistor;
NTC: Negative temperature coefficient thermistor.
When the ambient temperature rises, its resistance value decreases, and the use of electrical equipment or charging equipment can respond in time, control internal interruption and stop charging and discharging.
U1 is The circuit protection chip, U2 is two reversely connected MOSFET switches. Under normal conditions, both CO and DO of the battery board U1 output high voltage, and both MOSFETs are in the open state, and the battery can be charged and discharged freely.
Characteristics of lithium batteries
1. Long life
The cycle life of the long-life lead-acid battery is about 300 times, and the maximum is 500 times, while the lithium iron phosphate power battery has a cycle life of more than 2000 times. Lead-acid batteries of the same quality can be used 1 to 1.5 years at most, while the theoretical life of lithium iron phosphate batteries that used under the same conditions will reach 7 to 8 years, which means the performance-price ratio is theoretically more than 4 times that of lead-acid batteries.
High current discharge can charge and discharge quickly at a high current of 2C. With the matching charger, the battery can be fully charged within 40 minutes after charging at 1.5C, and the starting current can reach 2C, but lead-acid batteries do not have this performance.
2. No memory effect
The capacity of the battery will quickly drop below the rated capacity value when the battery is often fully charged, and this phenomenon is called the memory effect. Ni-MH and Ni-Cd batteries have memory effect, while lithium batteries do not. No matter what state the battery is in, it can be charged and used at any time, and there is no need to discharge it first before recharging.
3. High energy density
At present, the energy density of the mainstream lithium iron phosphate battery is below 200Wh/kg, and the energy density of the ternary lithium battery is between 200-300Wh/kg.
The lithium ion material used in the lithium battery has a higher energy density, which is generally higher than that of lead acid batteries have around three times the energy density, which means they are able to store more energy and therefore have a higher capacity in the battery of same size .
4. Light weight
The volume of the lithium iron phosphate battery with the same specification and capacity is 2/3 of the volume of the lead-acid battery, and the weight is 1/3 of the lead-acid battery.
5. Environmental protection
Lithium iron phosphate batteries are generally considered to contain no heavy metals or rare metals (rare metals are required for nickel-metal hydride batteries), non-toxic (SGS certified), non-polluting, and comply with European RoHS regulations, which is an absolute green battery certificate. So we can know whether are lithium batteries safe for the environment.
What are the risks of lithium batteries
Lithium batteries have a high energy density. Usually, the energy density of ternary lithium batteries reaches more than 200Wh/kg, and the higher the energy density, the worse the thermal stability. Once a thermal runaway reaction occurs, a high amount of heat will be released, which may be upgraded from spontaneous ignition to deflagration. So, are lithium batteries safe? The main reasons for the risks are as follows:
Over charge and over discharge
During normal use, electric energy and chemical energy are converted internally, but under conditions such as overcharge and overdischarge, it is easy to cause chemical side reactions inside the battery.
The side reaction will affect the performance and service life of the battery in the long run, and in severe cases, a large amount of gas may be generated, which will increase the pressure inside the battery and cause the problems of are lithium batteries safe such as explosion and fire.
Design flaw
On the one hand, design defects are reflected in the pursuit of excessively high energy density while ignoring safety. NCM811 battery is a typical example. The ratio of nickel, cobalt, and manganese in the cathode material is 8:1:1, although the proportion of nickel is increased.
The energy density is greatly improved, but the content of cobalt and manganese is reduced (cobalt can improve battery stability and extend battery life, and manganese can improve battery safety and stability), and the safety is greatly reduced.
On the other hand, the unreasonable design of the diaphragm in the battery structure, such as too short tab length, may cause potential safety hazards, or the compression of the thickness of copper foil and aluminum foil in order to save costs is also the reason for the increased concern of are lithium batteries safe.
Production process defects
In the production process of lithium batteries, due to process defects or lax control of the production process, impurities or excessive moisture in the battery are all dangerous behaviors, which will increase the side reactions of the battery, resulting in increased internal pressure during use and explosion and spontaneous combustion may occur.
What is the main safety concern with lithium-ion batteries
Are lithium batteries safe? Generally speaking, the problems of are lithium batteries safe are manifested as burning or even explosion. The root cause of these problems is the thermal runaway inside the battery. In addition, lithium-ion batteries may also be affected by some external factors and cause concer of are lithium batteries safe, such as overcharging, fire, extrusion, puncture, short circuit, etc.
According to statistics, 68% of electric vehicle lithium-ion battery fire accidents over the years were caused by internal or external short circuits, 15% were caused by charging and discharging, 7% were caused by accidental startup of equipment, and 10% were caused by other reasons.
From the perspective of the cause of the fire, electrical faults and spontaneous combustion are the main causes of fires in electric vehicles, while overcharging, battery cell failures, and short circuits in electrical circuits are the root causes of fires, and brings the concern of are lithium batteries safe.
Which battery is the safest
So are lithium batteries safe? The safety performance of lithium iron phosphate batteries will be higher than that of ternary lithium batteries. This is mainly because the heat resistance of lithium iron phosphate batteries is relatively good, and the thermal runaway temperature can reach more than 800℃.
The reason why the battery catches fire is mostly due to factors such as short circuit, impact, and overcharging, which drives to the convern of are lithium batteries safe. When the current passes through, a large amount of heat will appear in the overpotential and ohmic polarization, which will locally heat the battery to the temperature of the thermal decomposition of the cathode. The heat caused the battery to explode and catch fire.
The ignition point of the ternary lithium battery is only 200℃, and the ignition point of the lithium iron phosphate battery is 800℃. After an accident, the collision extrusion temperature can easily exceed the critical value.
In other words, lithium iron phosphate batteries will not spontaneously ignite if they do not reach 800℃. The ternary lithium battery is different. The thermal runaway temperature is basically around 200℃. Looking at the spontaneous combustion incidents of new energy vehicles, most of them are ternary lithium batteries. Therefore, for areas of hot weather, the risk of spontaneous combustion of ternary lithium batteries will be higher.
Should you wait for solid-state batteries?
From “liquid-state” to “solid-state”, the biggest advantage is to improve the problem of are lithium batteries safe. At the same time, solid-state battery can also achieve higher energy density, longer life, lighter weight and easier packaging, in short, for existing lithium batteries , there are many benefits of solid-state batteries. Despite their many advantages, solid-state batteries are still in the laboratory stage.
If it is to be mass-produced, there are still many problems to be solved in terms of production process, manufacturing environment, material selection and cost control. For example, the production environment needs to be extremely dry, and the dew point temperature should be below -40°C, and the processing of solid electrolytes is difficult.
Due to technical difficulties, the later the mass production of solid-state batteries, the higher the reliability. Although the forecast is not necessarily accurate, after summarizing the opinions of specialists, it can be judged that the semi-solid-state will achieve mass production. The more reliable time point is 2024/2025, and the full-solid-state batteries may be around 2030.
Semi-solid vs solid vs liquid lithium battery – compare their performance
| Test name | Test method | Semi-solid ternary | Liquid ternary | Liquid state LFP |
|---|---|---|---|---|
| Energy density (Wh / kg) | 0.33C | 230~250 | 230~250 | 183.4 |
| Over charge | And 2.5Umax times | Pass (10.6V) | Fail | Pass (9.1V) |
| Acupuncture | 8mm-25mm/s | pass | Fail | pass |
| Extrusion | 40% Deformation | pass | pass | Fail |
| Short circuit at Normal temperature | 25℃,1m Ω | pass | pass | pass |
| High temperature short circuit | 55℃:1m Ω | pass | pass | pass |
| Hot box | 30min | 180℃ | 130℃ | 200℃ |
| Charge / discharge at high and low temperatures | 0.33C 、1C : -30~55℃ | Excellent low temperature performance | good | Poor low temperature performance |
| Rate of charge / discharge | 0.33C-0.5C-1C-2C-3C | Liquid LFP uses carbon bottom coating foil, and the SP content in the formula is high | ||
| DCR at different temperatures | -30~55℃ | The liquid LFP did not complete the test | ||
| Test name | Summary of the semi-solid-state ternary comparison |
|---|---|
| Energy density (Wh / kg) | Semi-solid ternary ≥ liquid ternary > Liquid LFP |
| Over charge | Semi-solid ternary ≥ liquid ternary > Liquid LFP |
| Acupuncture | Semi-solid ternary ≥ liquid ternary > Liquid LFP |
| Extrusion | Semi-solid ternary ≥ liquid ternary > Liquid LFP |
| Short circuit at Normal temperature | Semi-solid ternary = liquid LFP = liquid ternary |
| High temperature short circuit | Semi-solid ternary = liquid LFP = liquid ternary |
| Hot box | Liquid LFP> Semi-solid ternary> liquid ternary |
| Charge / discharge at high and low temperatures | Semi-solid ternary ≥ liquid ternary > Liquid LFP |
| Rate of charge / discharge | Liquid LFP> Semi-solid ternary> liquid ternary |
| DCR at different temperatures | Semi-solid ternary ≥ liquid ternary > Liquid LFP |
Are solid-state lithium batteries safer than normal lithium batteries, and why?
Solid-state batteries do not use flammable electrolytes, which makes them worse concern of are lithium batteries safe. The solid-state battery uses a solid electrolyte on the channel where lithium ions move back and forth, instead of the liquid electrolyte of the existing lithium battery.
The electrolytic solution of the existing battery adopts organic solvent materials, which has the possibility of fire and has problems in safety. The safety of power batteries is closely related to liquid electrolytes, while solid-state batteries use solid-state electrolytes, which have the characteristics of good insulation, non-flammability, and non-volatility, which can significantly improve safety.
Comparison of performance of various power batteries
| Cell model | 12100319-50Ah | 13110310-50Ah | 12100319-50Ah |
|---|---|---|---|
| Scheme | Semi-solid ternary | Liquid state LFP | Liquid ternary |
| Energy density | 2 | 0 | 2 |
| Cycles | 1 | 2 | 1 |
| C-rate | 1 | 2 | 0 |
| Low temperature | 2 | 1 | 0 |
| DCR | 2 | 0 | 1 |
| Memory | 1 | 2 | 0 |
| Over charge | 2 | 2 | 0 |
| Acupuncture | 2 | 2 | 0 |
| Extrusion | 2 | 0 | 2 |
| Heat | 1 | 2 | 0 |
| Short-circuit | 2 | 2 | 2 |
| Comprehensive score | 18 | 15 | 8 |
Are solid lithium batteries safe – FAQs
What happens if you damage a lithium battery?
Are lithium batteries safe when damaged? When it happens, lithium ions and oxygen combine to produce a large amount of heat in a chemical reaction, which can cause the battery to catch fire or explosion. At this time, even if the battery does not collide, the heat generated by the battery due to the short circuit cannot be diffused, which may cause the lithium battery to burn, increasing the concern of are lithium batteries safe.
What happens when lithium touches water?
Are lithium batteries safe when water is immersed in the lithium battery? When it happens, it will cause corrosion of the internal electronic components, increase the local internal resistance, increase the heat generation, and affect the service life of the battery. In severe cases, the water will gradually corrode the charged protection board, resulting in a short circuit of the protection board and loss of protection.
At the same time, water will also corrode the battery core, and the electrolysis phenomenon will occur in the battery core, causing the battery shell to corrode and leak, and the leaked electrolyte will further corrode the protection board or circuit, resulting in battery failure or even fire accident.
Does temperature affect lithium batteries?
Lithium batteries will produce chemical reactions as the temperature changes. When the temperature decreases, the electrochemical reaction rate decreases and the internal resistance of the battery increases. Accompanied by the rapid increase of polarization internal resistance, the discharge capacity of the battery decreases, which affects the power and energy output of the battery.
Not only will it affect the performance of the lithium battery, but also the problem are lithium batteries safe. At the situation that the temperature of the battery is too high, and thermal runaway will occur. The battery will generate heat during charging and discharging. When the oxygen generated by the cathode reaches the anode, it will react with the lead in the anode. The reaction produces a large amount of heat.
If the battery continues to work in a high temperature environment, the heat accumulation inside the battery cannot be guided in time, which will cause the battery temperature to be too high, and the internal resistance will increase, gradually forming thermal runaway and causing the battery to fail.