Home » battery news » Causesanalysis of black spots on lithium battery anode
Causes analysis of black spots on lithium battery anode
During the use or storage of commercial lithium-ion batteries, due to the interaction of a series of complex chemical and physical mechanisms inside the battery, some failure phenomena often occur, which seriously reduces the performance, consistency, reliability and safety of lithium-ion batteries.
Among them, the failure of graphite-based anode materials mainly occurs on the surface of graphite. This article will analyze the cause of a black spots phenomenon on the anode surface of lithium-ion batteries.

What is black spots on the anode
The black spots on the anode occurs during the electrochemical reaction between the graphite surface and the electrolyte to form a solid electrolyte interfacial phase (SEI).
When the LFP cathode and graphite anode were first charged and discharged according to the conventional manufacturing process, it was found that the battery capacity was 0.1%~0.5% lower than the normal level, and the battery thickness increased by 1.85%.
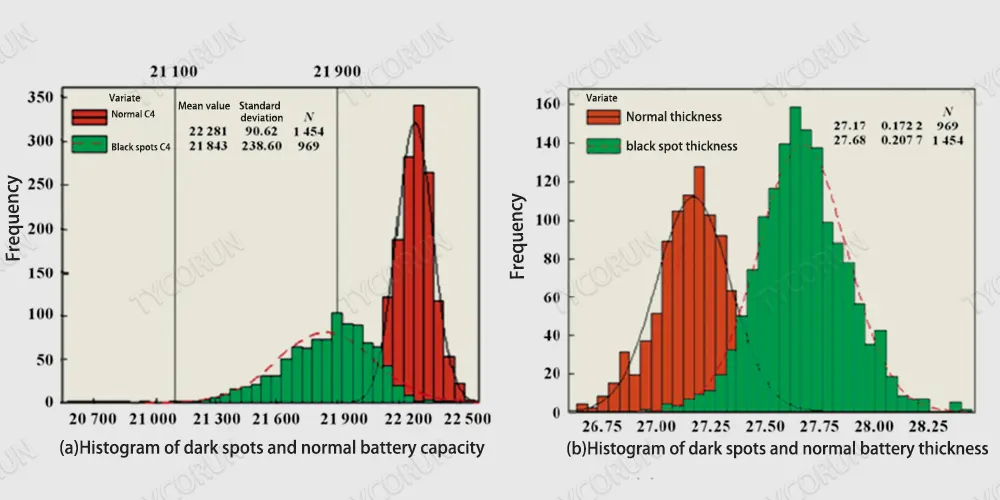
After 7 days of storage at high temperature [(55±2)℃] in the 100% SOC state, the charge and discharge battery test was performed again, and the battery capacity was attenuated to a certain extent and the dispersion became larger.
There were differences in the battery charge retention capacity, and the capacity retention rate was lower than normal. The normal level was 0.4%~0.9%, and the volume recovery rate was 0.7%~0.89% lower than the normal level.
Black spot and normal battery capacity recovery and capacity maintenance
| Number | Post-treatment C4 | Nominal capacity/Ah | Residual capacity after storage at (55±2)℃ for 7 days/Ah | Percentage of residual capacity/% | Recovery discharge capacity/Ah | Capacity recovery percentage/% |
|---|---|---|---|---|---|---|
| Black spot-3# | 22.062 | 21.43 | 20.79 | 97.00 | 20.97 | 97.85 |
| Black spot-4# | 22.010 | 21.42 | 20.86 | 97.40 | 20.93 | 97.71 |
| Normal-1# | 21.988 | 21.45 | 20.98 | 97.81 | 21.14 | 98.55 |
| Normal-2# | 21.981 | 21.41 | 20.97 | 97.94 | 21.11 | 98.60 |
After dissection of the battery, it was found that uneven black spots were produced on the anode pole surface of the battery with winding structure, and the degree of black spots was different for each pole group. Precipitation leads to an increase in the thickness of the anode sheet.

Element of black spots on the anode
The SEM&EDS composition analysis was carried out on the black spot position at the end of the pole piece and the normal pole piece.
The test results showed that: the composition of the normal negative pole piece was 100% C element, and the black spot position is except for 50%~96% C element (mainly from graphite material), also contains 4%~44% O element (mainly from the electrolyte), 1%~3% F/P element (mainly from the electrolyte salt LiPF6), and 2% Na element measured at individual points.
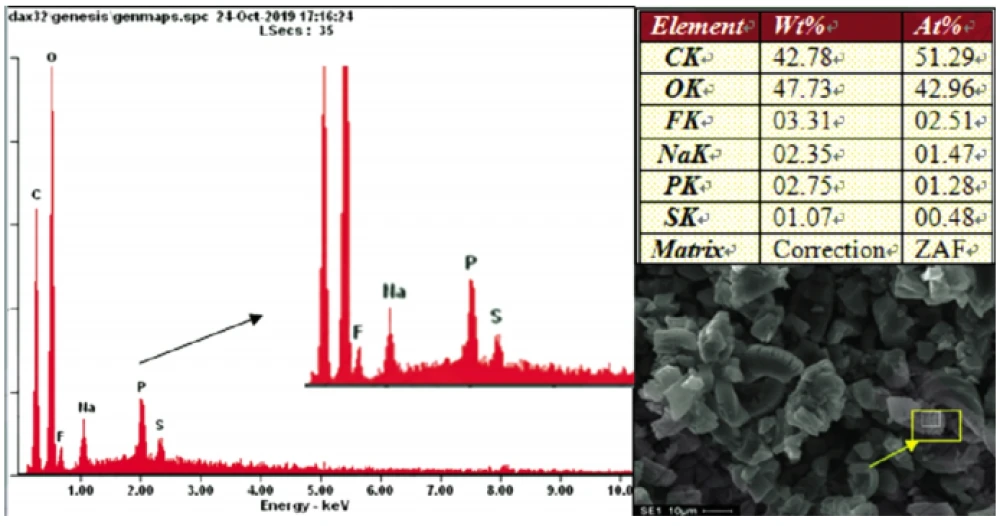
Through the electron microscope, it was found that the surface of the black spot anode was no longer dense, the graphite expanded, and the interlayer structure was destroyed. The inner graphite had many cracks caused by the splitting of graphite particles and abnormal holes caused by the failure of the powder-to-powder bond.
When observe the cross-section of the pole piece, it can be found that the black spot particles on the surface of the negative pole piece grow outward along the direction of the separator, and the stripping of graphite continues to consume electrolyte and active lithium, resulting in capacity dispersion and cycle performance degradation.
As the surface graphite expands and peels off, the fallen anode powder easily overlaps with the cathode sheet, triggering battery self-discharge.
Causes of black spots on the anode
The anode carbon materials have complex structures and various types, and their conductive behavior varies with different electrolyte systems. The failure of the anode surface is related to the structural characteristics of the carbon anode material, the composition of the electrolyte system, and the matching degree of the interface reaction between the anode and the electrolyte.
Structure of anode materials
There are many kinds of carbon materials used as anodes of lithium-ion batteries, and they have different structural characteristics with different raw materials and manufacturing processes. Therefore, when different types of carbon materials are used as anodes of lithium-ion batteries, their performance varies greatly.
Theoretically speaking, the better the layered structure of the carbon anode material, the more favorable it is for the insertion and extraction of lithium ions. Studies have shown that carbon materials with a higher degree of graphitization and the presence of SP3 hybridized carbon atoms can generate excellent SEI film and large lithium storage space.
Heat treatment at different temperatures can change the microstructure and graphitization degree of carbon materials. With the increase of heat treatment temperature, d002 gradually decreases, Lc gradually increases, lithium intercalation structure gradually increases, and graphitization degree also gradually increases.
Analyzing the black spot area of the negative sheet, it was found that although d002 and degree of graphitization remained unchanged, Lc and n decreased significantly, indicating that the graphite in this area had little or no lithium intercalation. SEM and EDX results are consistent.
Analysis and comparison of XRD data at different location
| Position |
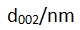
|

|

|
degree of graphitization |
|---|---|---|---|---|
| Black spot area | 0.3361 | 31.38 | 93 | 92.35 |
| Anode powder | 0.3359 | 53.11 | 159 | 94.17 |
The composition of electrolyte system
The reason why graphite materials can be used in lithium-ion batteries is due to the ion-conductive, electron-nonconductive solid state battery electrolyte interface film formed by the decomposition of the electrolyte on the graphite surface.
Its chemical composition and properties depend on the composition and properties of the anode material and electrolyte, which have an important impact on the performance and capacity of the battery.
At present, the organic solvents commonly used in the electrolyte of lithium-ion batteries for electric vehicles include EC, PC, DMC, DEC, EMC, etc. Some electrolyte decomposition products can form a stable SEI film, while some electrolytes will continue to undergo reduction and decomposition at a potential higher than that of lithium intercalation, eventually leading to the collapse of the graphite layer structure.
PC has a higher dielectric constant and a lower melting point, so electrolytes including PC have better performance at low temperatures. However, after PC solvent molecules are embedded in graphite, they cannot exist stably, but will undergo chemical reactions in the graphite host, unable to form a dense SEI film, and chemically decompose to generate CO, CO2, H2 and other gas phase by-products at the same time, resulting in graphite exfoliation and graphite exfoliation.
The cracking of the particles eventually causes the graphite structure to collapse, making it impossible to insert and remove lithium normally, resulting in poor cycle performance.
However, EC is in a solid phase at room temperature and cannot be used alone in traditional lithium-ion batteries. Its molecular structure is only one methyl group less than PC, and it also has a higher dielectric constant and better conductivity, but it is slightly higher than that of PC.
Decomposition occurs at a potential of 0.7V to form a stable SEI film, thereby inhibiting the decomposition of the electrolyte at a lower potential, so that lithium ions can be inserted and extracted normally in the graphite material, and the battery life is improved.
In addition, studies have shown that the lithium salt anion PF6- is the most fundamental reason for the difference in the interface behavior between PC and EC. When the graphite electrode voltage drops (that is, the battery charging process), since the volume of the solvated lithium ion solvation layer is much larger than the graphite layer spacing, a desolvation process needs to occur before being embedded in the graphite anode surface.
Electrolyte and active lithium are consumed in film formation and solvent co-intercalation, graphite particles actually have less active lithium embedded in the interlayer, and their macroscopic appearance appears as black spots.
Effect of Na content
From the EDX composition test, it can be seen that the main component of black slag is C, in addition to containing 2% Na element. In a small current, especially in a charged stock state, CMC (carboxymethyl cellulose) can undergo cation exchange reactions and ionize to form Na ions.
According to theoretical calculation, the theoretical content of Na element in CMC is equivalent to the content of Na element in ICP test anode powder. Therefore, it is speculated that the Na element is more likely to come from CMC, and the lack of moisture control inside the battery is the main cause of black slag in the anode sheet.
ICP test data of anode powder at black spots
| Sample | Element content in the sample | ||||
|---|---|---|---|---|---|
| Anode powder at black spots | Ca | Na | Fe | Cu | Li |
| 535.3 | 1346.1 | 45.1 | 75.3 | Over limit | |
In the process of charging and discharging with a small current, the migration rate of ions in the electrode is greater than the reaction rate of the ion intercalation electrode, so the process of ion intercalation into graphite is controlled by the intercalation electrode reaction, and the ion intercalation electrode reaction rate is proportional to the concentration of metal ions on the electrode surface.
Because Li+ has a small radius and a particularly large charge density, it has a strong ability to polarize solvent molecules, that is, the binding energy with solvent molecules is large, and Li+ interacts with the oxygen atoms on the polycyclic ring of solvent molecules through the electrostatic force of ion dipole moment.
In combination, in addition to forming the first layer of solvent layer, it may also form a second layer of solvent layer with more solvent molecules, which leads to the solvation radius of Li+ being much larger than Na+, and the ion concentration is lower than Na+.
Therefore, the embedding rate of Li+ in the electrolyte is smaller than that of Na+, resulting in a higher concentration of Na+ on the electrode surface, which can be embedded faster and form graphite interlayer compounds on the electrode surface, resulting in the internal expansion of the negative graphite particles and the formation of black spots.
In addition, due to the ionization of CMC, sodium salt is introduced into PC solvent to form [NaPC]+ complex, whose LUMO orbital energy is much lower than that of pure PC molecule. This suggests that [NaPC]+ complexes are more easily reduced than PC molecules.
The hybridization of LUMO orbitals between the HOMO orbitals of PC molecules and Na+ causes the LUMO orbital energy of [NaPC]+ complexes to decrease, resulting in the decomposition of PC solvents, gas production, and finally the spallation of negative graphite and structural collapse.
Effect of water
The water contained in the organic electrolyte will react with the organic solvent in it to produce the alcohol, followed by the organic electrolyte containing water in the battery’s first charging and discharging process, the following reaction will occur on the C anode:
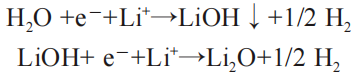
At the same time, the large amount of gas generated in the reaction will also lead to the increase of the internal pressure of the battery, and eventually lead to the stripping of the anode graphite and the collapse of the structure.
The presence of water in the organic electrolyte can also cause the hydrolysis of LiPF6 to produce HF and POF3. On the one hand, HF can play a certain catalytic role in the hydrolysis of LiPF6, further consuming the limited Li+ in the battery and accelerating the deterioration of the electrolyte. At the same time, it can catalyze the polymerization of organic solvents, resulting in increased viscosity and decreased conductivity of organic electrolyte.
In addition, as mentioned above, when there is a small amount of water or weak acid, CMC can undergo cation exchange reaction and ionize to form Na+. Na+ can be embedded faster than Li+ and form graphite interlayer compounds on the electrode surface, resulting in internal expansion of the negative graphite particles.
Deterioration condition of black spots on the anode
The performance of lithium-ion battery is greatly affected by the dynamic characteristics. During the storage process, the anode is at a low potential for a long time, which will cause the electrolyte to decompose continuously on the surface of the graphite anode and consume active Li, which is the main factor for the reversible capacity loss of lithium-ion battery during the storage process.
The SOC of the battery and the ambient temperature and other factors will affect the decomposition rate of the electrolyte on the anode surface, which will significantly affect the decline rate of the reversible capacity of the battery during the storage process.
When stored at low temperature (0℃ or below) or charged and discharged at low temperature, the electrochemical polarization of the anode is more obvious, the dynamic characteristics of the graphite anode are deteriorated, the electrode sheet is relatively brittle and the elasticity is not good.
And the active material may deform in the lithium space during the charging and discharging process of the electrode sheet at low temperature, which will affect the stability of the conductive network of the electrode sheet and is not conducive to the conduction of Li+. The side effects are aggravated and the dark spots will be worsened.
Conclusion
The low degree of graphitization of the anode sheet or the small graphite layer spacing affect the graphite without lithium or less lithium. The co-intercalation reaction occurs between the PC component of electrolyte and the active lithium consumed in film forming and solvent, which affects the intercalation of the active lithium between graphite particle layers.
The residual water in the cell dissociates CMC to form Na+ complex, which leads to the intensification of graphite intercalation reaction. At the same time, the residual water causes the electrolyte to produce gas and decompose, resulting in the graphite stripping of the anode sheet.
The combined effect of these conditions is the main reason for the formation of black slag in the anode sheet. And low temperature storage or low temperature (0℃ and below) charge with battery charger and discharge will lead to the difficulty of removing lithium and the aggravation of side reactions, and the deterioration of black spots.