Graphene application potential - application of graphene in lithium batteries

Graphene has ultra-high electron mobility, specific surface area and tensile strength, as well as excellent flexibility. It can not only improve the energy and power density of lithium batteries, obtain higher lithium storage capacity and better fast charging performance, but also help overcome the solid/solid interface technical problems of solid state battery.
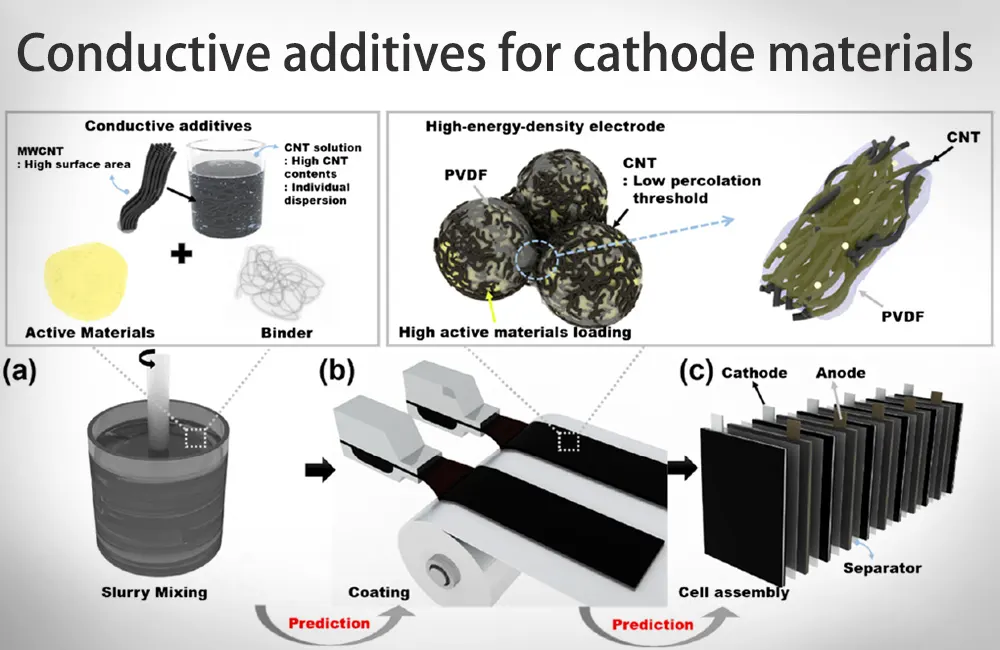
Conductive additives for cathode materials
At present, lithium nickel cobalt manganate (ternary lithium battery) and lithium iron phosphate (lifepo4 battery) are the most commonly used cathode materials for lithium-ion batteries. Their advantages are good cycle performance and high theoretical specific capacity.
However, the poor conductivity of these materials makes the internal resistance of the electrode larger, which seriously affects the cycle, rate and safety of the battery. Therefore, it is necessary to improve its performance by adding conductive agents.
The basic function of the conductive agent material is to establish a high-speed ion transport channel between the electrode active particles to increase the electron transport speed. Carbon-based materials have light weight, high electrical conductivity, thermal conductivity, and good chemical stability, and are currently the most widely used type of conductive agent.
Traditional conductive agents such as conductive graphite and conductive carbon black in carbon-based materials have been unable to meet market demand, so research and development of new conductive agents is imperative.
● Graphene single-phase conductive agent
As a two-dimensional nanomaterial, graphene has excellent electrical conductivity. Graphene with a large specific surface area is attached to the surface of the cathode material particles, and intertwined with each other to form a huge high-speed conductive network. The migration rate of lithium ions and electrons can be effectively improved.
Therefore, the composite material composed of graphene with high electrical conductivity and large specific surface area and the cathode material of lithium battery can overcome the shortcomings of insufficient electrical conductivity of the electrode material, and give full play to the characteristics of high specific capacity.
In the case of charging and discharging batteries with a small rate, as the amount of graphene added increases, the discharge specific capacity first increases and then decreases. Compared with commercial conductive agents, adding a small amount of graphene can achieve a good conductive effect, and the battery performance is also improved accordingly.
However, when the battery is charged and discharged at a high rate, the performance of using graphene alone as a conductive agent is not as good as that of conventional conductive agents. So people began to study the combination of graphene and conventional conductive agents to form binary conductive agents and add them to batteries.
● Graphene and carbon black composite conductive agent
When graphene and carbon black are constructed as a composite conductive agent material, a large number of two-dimensional graphite sheets are evenly wrapped on the basis of the original network chain carbon black structure, and the gaps between the sheets are filled with carbon black that acts as a skeleton structure.
Through the synergistic conduction, the original two-dimensional conduction at the point is changed to the three-dimensional structure conduction at the point, and at the same time, the problem of graphene superposition and agglomeration is solved, and the structural stability and conduction efficiency are improved.
● Graphene and carbon nanotube composite conductive agent
When graphene/carbon nanotubes are added as conductive additives to lithium-ion battery materials, the two can build a three-dimensional network of conductive sites. Carbon nanotubes run through each layer of graphene sheets, turning the original two-dimensional conduction space into a three-dimensional bridging structure transport channel.
It provides a faster and smoother electron conduction path, which greatly improves the transmission efficiency and the transmission rate of lithium ions in the cathode of the battery. At the same time, the skeleton effect of carbon nanotubes effectively enhances the stability of the graphene structure, avoiding agglomeration and stacking .
Composite anode material
Graphene can be added to the anode materials of lithium batteries to enhance their electrochemical performance. Conventional lithium batteries in top 10 lithium ion battery manufacturers use anode materials such as graphite, which have low specific capacity and energy density and cannot meet the new needs of the market.
At present, the research on anode materials is mainly focused on Si-based materials, lithium metal materials, transition metal oxides/sulfides, etc., which can promote battery capacity. However, these materials generally have problems such as large volume change and poor conductivity, which restrict the performance of lithium batteries.
Combining graphene with these new anode materials to construct various coating structures is an effective way to solve this problem.
● Silicon and graphene composite anode material
On the one hand, the structure of graphene has certain mechanical strength and flexibility, which can buffer the volume expansion of silicon during the lithiation process. It helps to improve the conductivity of silicon, so as to obtain a silicon/graphene composite electrode material with better performance.
On the other hand, the incorporation of graphene makes the dispersion of silicon nanoparticles more uniform, which is conducive to the improvement of material cycle performance and specific capacity.
● Lithium metal and graphene composite anode materials
Graphene has strong mechanical properties, good flexibility and chemical corrosion resistance, and can adapt to the volume change and complex electrochemical environment of lithium metal during battery cycling. The use of graphene-modified lithium anode is expected to achieve the protection of lithium metal.
The study found that graphene needs to have a porous structure and abundant lithiophilic sites in order to stabilize the SEI film and effectively inhibit the growth of lithium dendrites. At the same time, the number of stacking layers will affect the degree of bonding between graphene and lithium and the morphology of graphene in the composite material, thereby affecting the electrochemical performance of graphene/lithium metal composite materials.
● Metal oxide and graphene composite anode materials
When pure metal oxide is used as the battery anode material, the specific capacity can reach 700-1000mAh/g, but in the process of charging and discharging, it is prone to volume expansion, and the battery capacity and rate performance will also decrease accordingly.
Graphene is filled between metal oxide particles, which can not only slow down the volume change of metal oxides, but also effectively enhance the conductive effect with good electrical conductivity and large specific surface area, increase the Li+ transmission rate, and significantly improve the electrical conductivity.
Embedding metal oxides into graphene builds a new three-dimensional transport structure, which makes the structure of the composite material more stable, effectively reduces the phenomenon of agglomeration and superposition during charging and discharging, and further improves the storage capacity of the battery.
Interface modification of solid state batteries
At present, the research on high-conductivity solid-state electrolytes has made significant progress, but the wettability of the solid/solid interface formed with electrodes is poor, which has adverse effects on interfacial resistance, chemical compatibility and interfacial stability.
In the anode interface region, the construction of three-dimensional graphene frameworks, laminates, and hollow spheres can inhibit the formation of lithium dendrites and the volume expansion of the electrode during charging.
In the cathode interface region, graphene is compounded with electrode materials and electrolytes to improve charge transfer. In addition, graphene acts as a buffer layer to improve the interfacial compatibility and thus improve the overall performance of the battery.