Battery grade lithium hydroxide introduction and comparison

Then to the downstream traditional industries (metal smelting, lubricants, ceramic glass, etc.), new materials (organic synthesis, biomedicine) and new energy (3C batteries, power batteries, etc., for major 3C lithium battery manufacturers.
could refer to top 10 3C consumer lithium battery manufacturers.) and other application-side complete industrial chains. This article will introduce the production process of lithium hydroxide, a lithium battery material, and the comparison of analysis of lithium carbonate.
Introduction of battery grade lithium hydroxide
Lithium hydroxide is one of the three basic lithium salts in the lithium industry chain, and its main forms mainly include anhydrous lithium hydroxide (LiOH) and monohydrate lithium hydroxide (LiOH·H2O). Battery-grade lithium hydroxide monohydrate is mainly used in the preparation of positive electrode materials for lithium-ion batteries. It can also be used as an additive for alkaline battery electrolytes, and is also used in the manufacture of lithium.
Lithium hydroxide, as the core lithium salt species downstream of the lithium industry chain, is an important raw material in the field of power batteries, especially the high-nickel ternary cathode material widely used in high-performance power batteries, and is an indispensable core lithium source in its production.
High-nickel ternary materials in the field of power batteries are mainly divided into NCM811 and NCA. Chinese companies mainly produce NCM811, and Japanese and Korean companies mainly produce NCA. For specific Chinese manufacturers of NCM811 materials, please refer to top 5 NCM811 high nickel ternary cathode material companies in China. At present, many new energy vehicles equipped with high-nickel ternary batteries have a battery life of more than 500km.
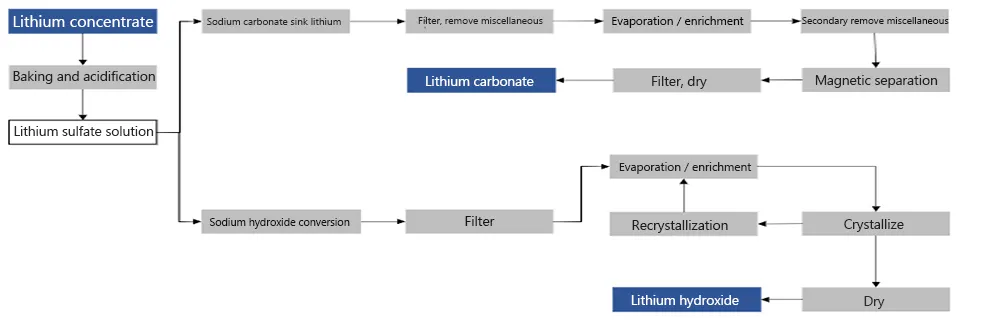
Production process of lithium hydroxide
From the perspective of production technology, lithium hydroxide can be produced by one-step method after lithium sulfate is produced through acidification, or crude lithium carbonate can be produced first by extracting lithium from salt lake brine, and then causticized to produce hydrogen lithium oxide. Therefore, considering the cost of producing lithium hydroxide from spodumene is more advantageous.
Since the salt lake system has a clear cost advantage in lithium carbonate, and the ore system has a quality advantage and no cost disadvantage in lithium hydroxide, the new production capacity of lithium compounds in the ore system since 2019 is mainly concentrated in the battery-grade lithium hydroxide product line.
However, the new production capacity of mica lithium extraction is concentrated in the lithium carbonate product line, and its “quasi-battery-grade” products form a supplement to lithium extraction from salt lakes.
Lithium extraction from ore
The main disadvantage of extracting lithium from ores is that, except for a few high-grade resource sites (such as the Talison-Greenbush lithium mine), the use of ores to produce lithium carbonate and lithium chloride products is generally in the middle to high position of the global cost curve.
From the perspective of cost safety margin, lithium extraction from ore is more suitable for the production of high-quality products such as battery-grade lithium hydroxide.
Lithium extraction from salt lake brine
As far as the current mature technology is concerned, lithium extraction from salt lakes is more suitable to be positioned as a low-cost raw material base for the production of basic lithium salts such as lithium carbonate (industrial grade/battery grade) and lithium chloride.
The production of lithium hydroxide requires causticization on the basis of lithium carbonate products. But in the future, if electrolysis, bipolar membrane and other processes become mature, it will help Salt Lake to achieve one step and directly produce high-quality lithium hydroxide products.
Comparison of lithium carbonate vs lithium hydroxide
Both lithium carbonate and lithium hydroxide are raw materials for batteries. In the market, the prices of lithium carbonate and lithium hydroxide basically keep rising and falling at the same time. What is the difference between these two materials?
Comparison from preparation process
Both can be extracted from spodumene, and the cost is not much different, but if the two are transformed into each other, additional costs and equipment are required, and the cost performance is not high. The technical routes are different. The preparation of lithium carbonate mainly adopts the “sulfuric acid method”. Lithium sulfate is obtained through the reaction of sulfuric acid and spodumene, sodium carbonate is added to the lithium sulfate solution, and then separated and dried to prepare lithium carbonate;
Lithium hydroxide is mainly prepared by “alkali method”, that is, it is prepared by roasting spodumene and calcium hydroxide. Some also use the so-called sodium carbonate pressurization method, that is, first prepare a lithium-containing solution, then add lime to the solution, and then prepare lithium hydroxide.
In short, spodumene can be used to prepare both lithium carbonate and lithium hydroxide, but the process paths are different, the equipment cannot be shared, and there is not much difference in cost. In addition, the cost of preparing lithium hydroxide from salt lake brine is much higher than that of lithium carbonate.
The technical difficulty of converting lithium carbonate into lithium hydroxide is small, but the cost and construction period are relatively troublesome. The “causticization method” is used to prepare lithium hydroxide from lithium carbonate. It participates in the reaction of calcium hydroxide in lithium carbonate to produce lithium hydroxide. The process is relatively sophisticated.
However, it is necessary to build a special production line. The production cost per ton is at least 6,000 RMB without considering depreciation and other factors. Considering factors such as environmental impact assessment, the construction period is at least 1-2 years. When the lithium carbonate price is higher than the lithium hydroxide price, the lithium carbonate causticization method will directly sell lithium carbonate without further producing lithium hydroxide.
It is simpler to prepare lithium carbonate from lithium hydroxide, but additional costs are also required. Add carbon dioxide to lithium hydroxide solution to obtain lithium carbonate solution, and then separate, deposit, and dry to obtain lithium carbonate. Similarly, this process requires the construction of a dedicated production line, which also requires additional additional costs.
Comparison from applicability
Since high-nickel ternary batteries require a lower sintering temperature, lithium hydroxide has become a necessary lithium salt for the preparation of high-nickel ternary materials. The preparation of lithium iron phosphate (LFP) products by hydrothermal method also requires the use of lithium hydroxide.
NCA and NCM811 must use battery-grade lithium hydroxide, while NCM622 and NCM523 can use either lithium hydroxide or lithium carbonate. Generally speaking, the performance of products produced by using lithium hydroxide is more excellent. Specifically:
Sintering temperature: The sintering temperature of ternary cathode materials of series 8 and above is usually low, if lithium carbonate is used as the lithium source, it is easy to cause incomplete decomposition due to insufficient calcination temperature, too much free lithium on the surface of the positive electrode, too strong alkalinity, and increased sensitivity to humidity;
Therefore, high-nickel ternary cathodes usually use lithium hydroxide as the lithium source. The use of lithium hydroxide and lower sintering temperature can also reduce cation mixing and improve cycle stability. In contrast, the sintering temperature of lithium carbonate often needs to be above 900°C to obtain a material with stable properties, and it is difficult to be used as a lithium source for high-nickel ternary materials.
Discharge capacity/tap density: Lithium hydroxide is used as the lithium source material, the first discharge capacity is as high as 172mAh/g, and it has better tap density and higher charge and discharge performance.
Consistency: Compared with lithium carbonate, lithium hydroxide has the advantages of good stability and good consistency, and is more suitable for high-end cathode materials.
Cycle life: The ternary material prepared with lithium hydroxide as the lithium source has more uniform particles, which can greatly improve the cycle life of the ternary material.
In general, battery-grade lithium hydroxide is better than lithium carbonate for ternary materials.