Home » battery news » The influence of lithium precipitation and SEI film on lithium battery capacity degradation
The influence of lithium precipitation and SEI film on lithium battery capacity degradation

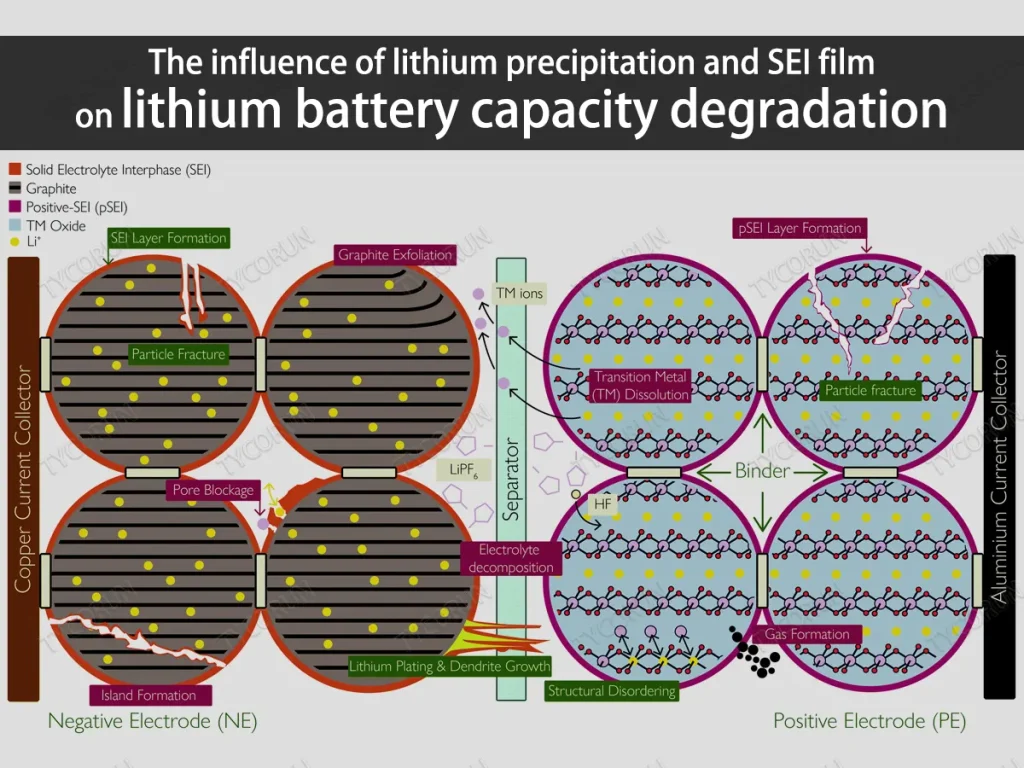
Causes of lithium-ion battery aging
The aging process of lithium-ion batteries is affected by many factors such as the way they are assembled as power wheels battery, ambient temperature, charge and discharge rates, and depth of discharge. Capacity and performance decline are usually the result of a variety of side reaction processes, and are related to many physical and chemical mechanisms, and its attenuation mechanism and aging form are very complex.
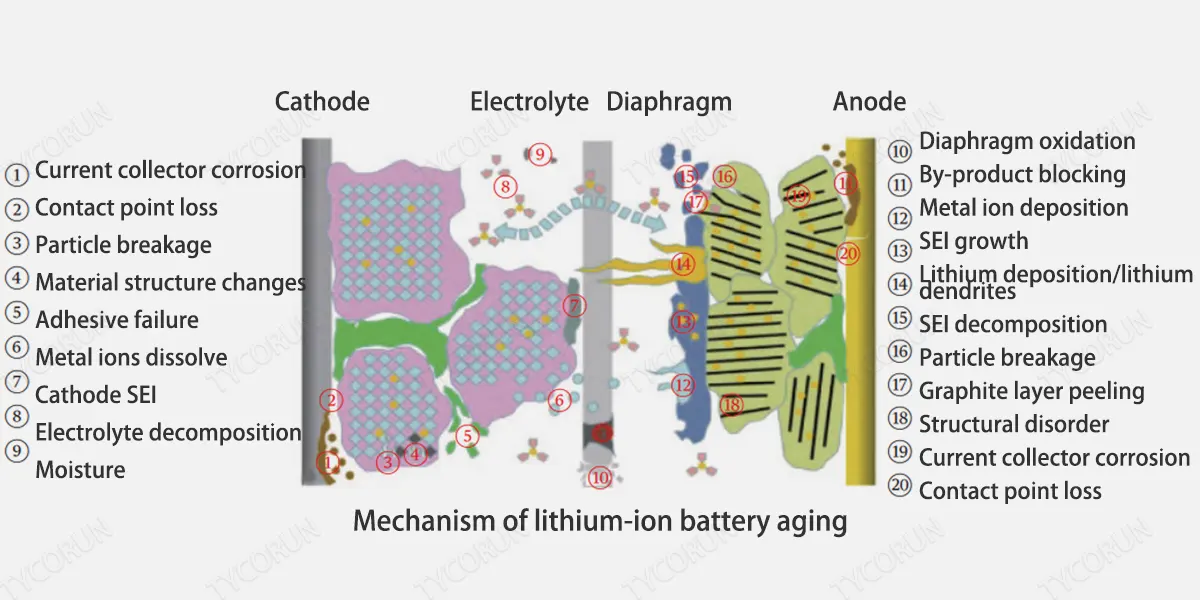
The figure below shows a comprehensive mechanism analysis of lithium-ion battery aging. During the actual aging process of lithium-ion batteries, different side reactions or phase change processes will occur in each component of the lithium-ion battery, and various processes have different effects on capacity degradation.
Based on global research progress in recent years, currently the main factors affecting the capacity degradation include: SEI film growth, electrolyte decomposition, battery self-discharging, electrode active material loss, current collector corrosion, etc.
In the actual aging process of lithium-ion batteries, various side reactions occur simultaneously with electrode reactions, and various aging mechanisms work together and couple with each other, making it more difficult to study the aging mechanism.
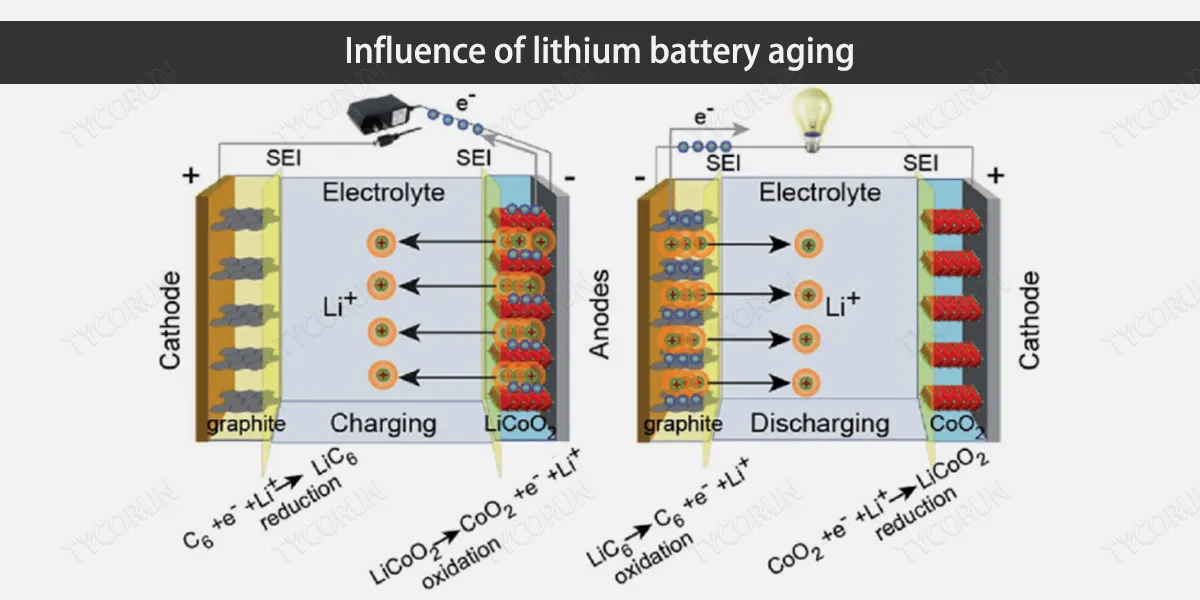
Influence of lithium battery aging
The aging of lithium-ion batteries has a profound impact on the overall performance of the battery, which is mainly reflected in the decline in charge and discharge performance, available capacity attenuation, and thermal stability.
The main external characteristics of lithium-ion batteries after aging are the decrease in available capacity and the increase in battery internal resistance, which in turn leads to a decrease in the actual charge and discharge capacity and maximum available charge and discharge power even for the best rechargeable batteries.
At the same time, due to the increase in internal resistance of lithium-ion batteries, problems such as increased heat generation, temperature rise within the module, and increased temperature inconsistency have increased the requirements for the thermal management system of lithium-ion batteries.
Side reactions within lithium-ion batteries are caused by the battery grouping method and connection structure, which leads to the differences in the usage conditions of the cells. As the battery is used, the aging speed of each cell in the battery is different, exacerbating the inconsistency of the lithium-ion battery pack.
The open circuit voltage curve of a lithium-ion battery represents the current internal electromotive force of the lithium-ion battery. As the lithium-ion battery ages, the open circuit voltage curve will shift or deform to a certain extent relative to the original state, causing the actual charge and discharge voltage curve of the lithium-ion battery to change, affecting the battery management system during actual use.
As the lithium-ion battery ages, the maximum charge-discharge rate available for the lithium-ion battery will also decrease. If the battery management system does not make adaptive adjustments, it is easy for the lithium-ion battery to be overcharged, overdischarged, and over-powered, increasing the safety risks of using lithium-ion battery.
Influence of lithium precipitation on battery capacity degradation
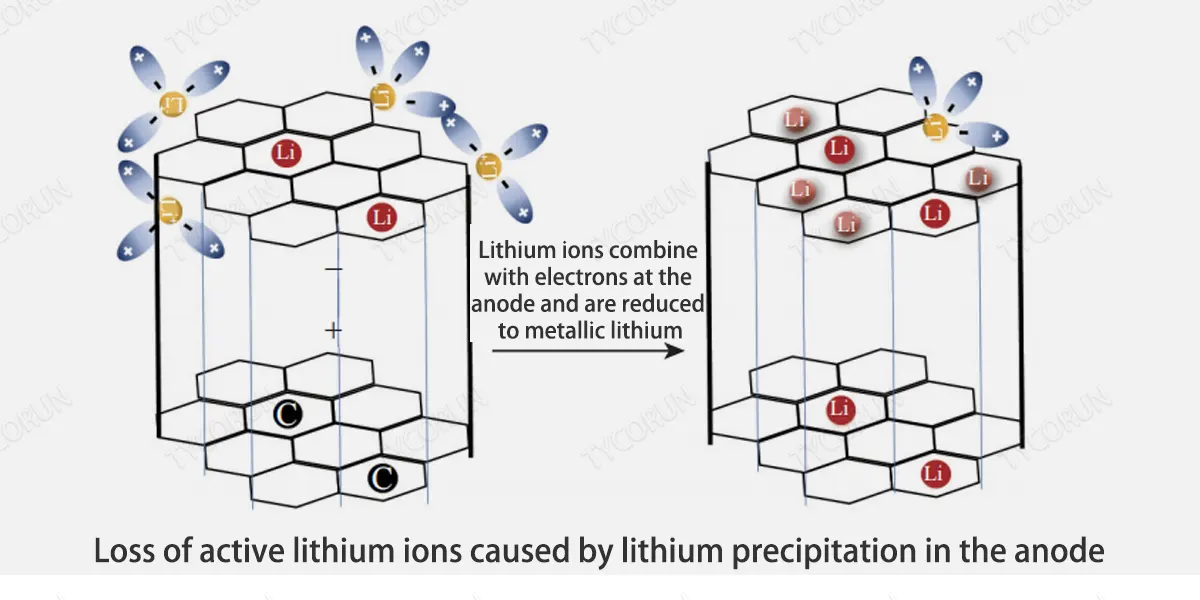
The figure below shows the loss of active lithium ions caused by lithium precipitation in the anode. lithium precipitation refers to the process of lithium precipitation from the electrolyte to the electrode surface.
Lithium precipitation on the surface of the anode is an important cause of aging of lithium-ion batteries and an important factor affecting battery safety. When the anode potential exceeds the threshold of 0V (relative to Li/Li+), lithium precipitation will occur on the anode surface.
There are many factors that affect lithium precipitation in batteries. Some researchers believe that the rate at which lithium ions are embedded in the graphite anode is too slow or the rate at which lithium ions are transferred to the anode is too fast, which may cause lithium precipitation.
Other studies have shown that the diffusion rate of lithium ions will slow down when working under low temperature conditions, and the working potential of the anode is very close to the lithium precipitation potential, making it easier to cause lithium precipitation.
In addition, if N/P (the ratio of the capacity of the anode sheet to the capacity of the cathode sheet) is too small, it may also lead to lithium precipitation.
Lithium precipitation is closely related to the battery aging. Some researchers believe that electrode lithium precipitation is more likely to occur in batteries with internal defects.
Some studies and battery test have also found that battery lithium precipitation will accelerate in the later stages of aging, thus becoming one of the main reasons for the inflection point of battery capacity.
The reason is that as the battery ages, the SEI generation causes the porosity of the anode to decrease, and the gradient of the electrolyte potential at the anode increases. Therefore, the anode potential decreases during the charging process, and it is easier to drop below 0V and lithium precipitation occurs.
The process of lithium precipitation will lead to the decrease of the porosity of the anode and the increase of the electrolyte potential gradient, which will accelerate the aging of the battery.
When the battery is in the discharge state, the lithium on the dendrites may dissolve out, but this part of the substance cannot get electrons because it is not in contact with the collector fluid, and cannot participate in the electrode reaction during the charge and discharge process, forming dead lithium. Lithium deposition leads to the loss of active lithium ions as shown in the figure below.

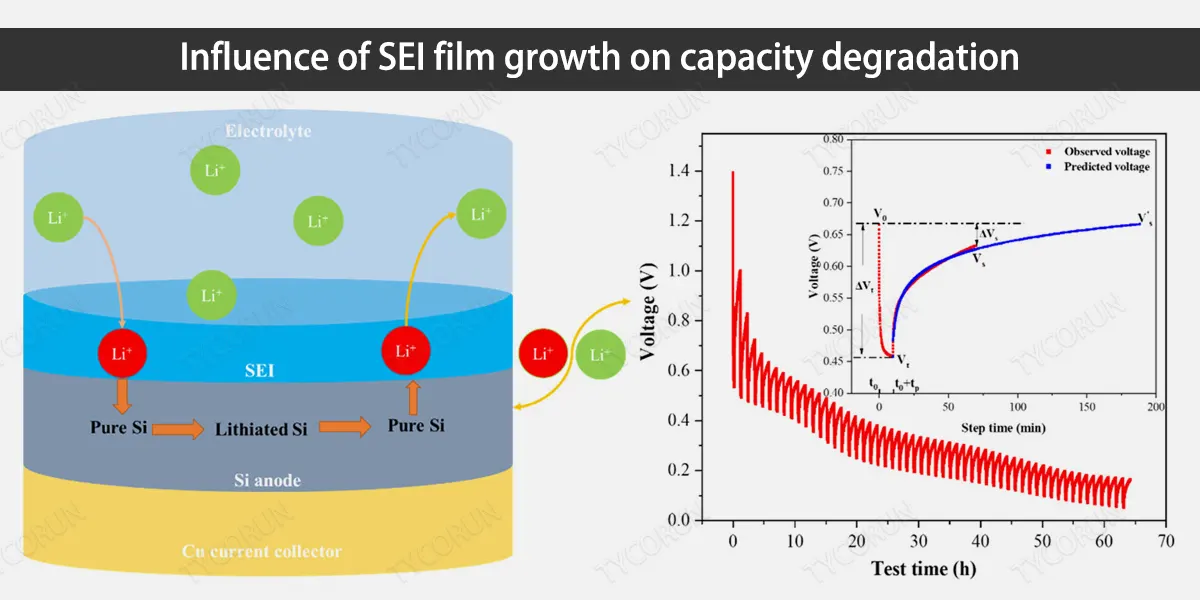
Influence of SEI film growth on capacity degradation
The SEI film is a passivation film formed on the surface of the anode of the lithium-ion battery, which has ion conductivity and prevents the passage of electrons, separating the electrolyte from the anode. SEI film growth is the main side reaction of lithium-ion battery at the anode/electrolyte interface, which leads to irreversible capacity loss.
Battery power, life and safety characteristics are closely related to SEI film. Under normal conditions of use, SEI film is the main factor that causes the loss of active lithium in batteries.
The SEI film is mainly composed of inorganic substances such as Li2CO3, LiF, Li2O and organic substances such as ROCO2Li, ROLi, RCOO2Li (R is an organic group). For some batteries, the thickness of the SEI film can reach more than 100nm.
The charge and discharge process of lithium-ion battery is accompanied by the repeated release and insertion of lithium ions between cathode and anode. During charging with battery charger, the active lithium ions in the cathode material will pass through the diaphragm to the anode surface, and the semi-battery reaction will be embedded in the anode material.
Since the working potential of the anode surface of the lithium ion battery is generally lower than the thermodynamically stable potential window of the electrolyte.
Once the lithium ion, the electrolyte and the electrons on the anode surface contact, the possibility of the electrolyte will be reduced, coupled with the complex reaction between various substances near the anode, resulting in the formation of SEI film on the cathode surface, resulting in the loss of the active material of the lithium ion battery.
SEI film formation is also one of the main causes of calendar aging at higher temperatures and higher state of charge (SOC). Compared with new batteries and SEI films generated under normal temperature cycles, SEI films generated at higher temperatures have better thermal stability and higher density than SEI films generated at lower temperatures, which can slow down the aging of batteries.
Although the growth of the anode SEI film will have a negative impact on the capacity and internal resistance of the lithium-ion battery, a stable SEI film can improve the interface properties of the electrode material and help improve the battery cycleability.
Some researchers also believe that the double-layer structure formed by the dense inner layer (initial SEI film) and the porous outer layer (long-term growth layer) of the SEI film can better explain the impact of the SEI film on battery characteristics.
Although the composition of the SEI film is still difficult to accurately analyze, the growth, rupture, and regeneration process of the SEI film are considered to be inseparable from the battery capacity degradation process.
The SEI membrane is produced when it is first formed. At this time, the SEI membrane is loose and porous. The electrolyte penetrates through the pores on the membrane surface and decomposes when in contact with the electrode. The product fills the pores, causing the SEI membrane to become dense.
However, during the long-term use cycle of the battery, the electrode material itself also undergoes expansion, rupture and other phenomena, causing the SEI mold on the surface to bear stress and become thinner, causing the SEI film to continue to grow during the cycle.
However, the SEI film will also be damaged during the rapid discharge process. During this process, the volume of the electrode shrinks rapidly, and the SEI film undergoes large stress and ruptures, causing the failure of the SEI film. The ruptured SEI film is gradually repaired in the subsequent cycles. However, partial ruptures will cause the overall structure of the SEI film to be irregular, causing swollen battery in severe cases.
The current density near the growth part is larger, forming a positive feedback that accelerates the growth of the SEI film in this part, resulting in partial abnormal aging and gradually causing the overall capacity decline of the battery.
Reasonable formation technology will improve the density of the SEI film and slow down the aging process. At the same time, low temperature environments are also conducive to the formation of dense SEI films, thereby extending the battery life.